Carbamazepine
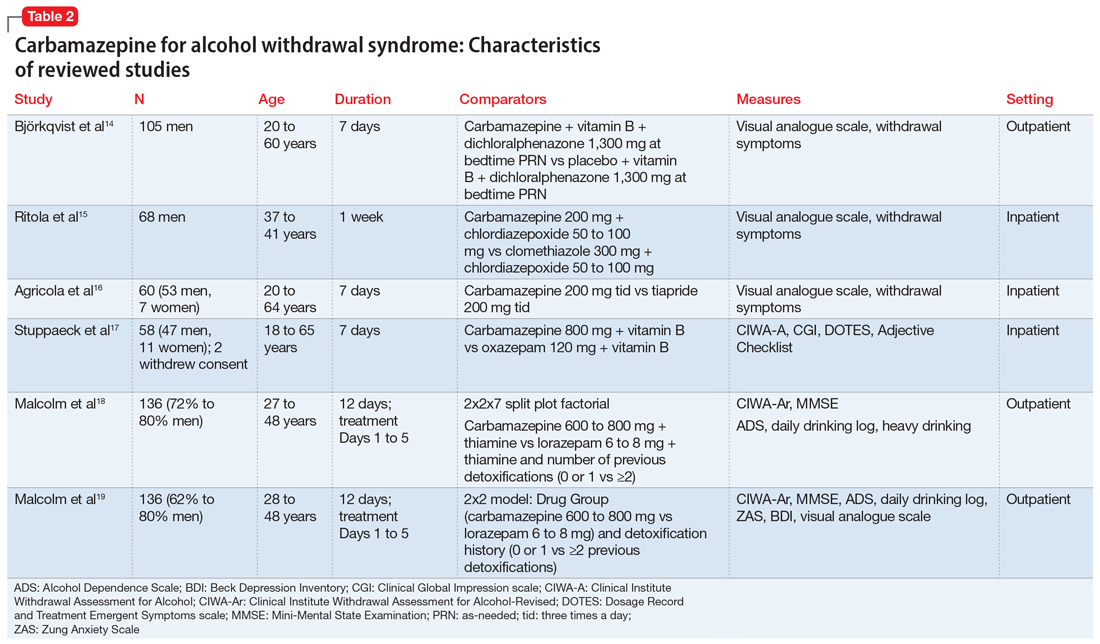
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19
Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al