Esketamine was evaluated in a randomized, placebo-controlled, double-blind, multicenter, short-term (4-week) phase III study in adult patients age 18 to 65 with TRD (they had not responded to at least 2 different antidepressants of adequate dose and duration).4 After discontinuing prior antidepressant treatments, all patients were started on a newly initiated antidepressant and were also randomized to concomitant intranasal esketamine or intranasal placebo as follows:
- 114 patients were randomized to the intranasal esketamine plus newly initiated oral antidepressant arm
- 109 patients were randomized to the placebo nasal spray plus newly initiated oral antidepressant arm
- The mean baseline Montgomery-Åsberg Depression Rating Scale (MADRS) score for each group was 37 (ie, moderately to severely depressed).
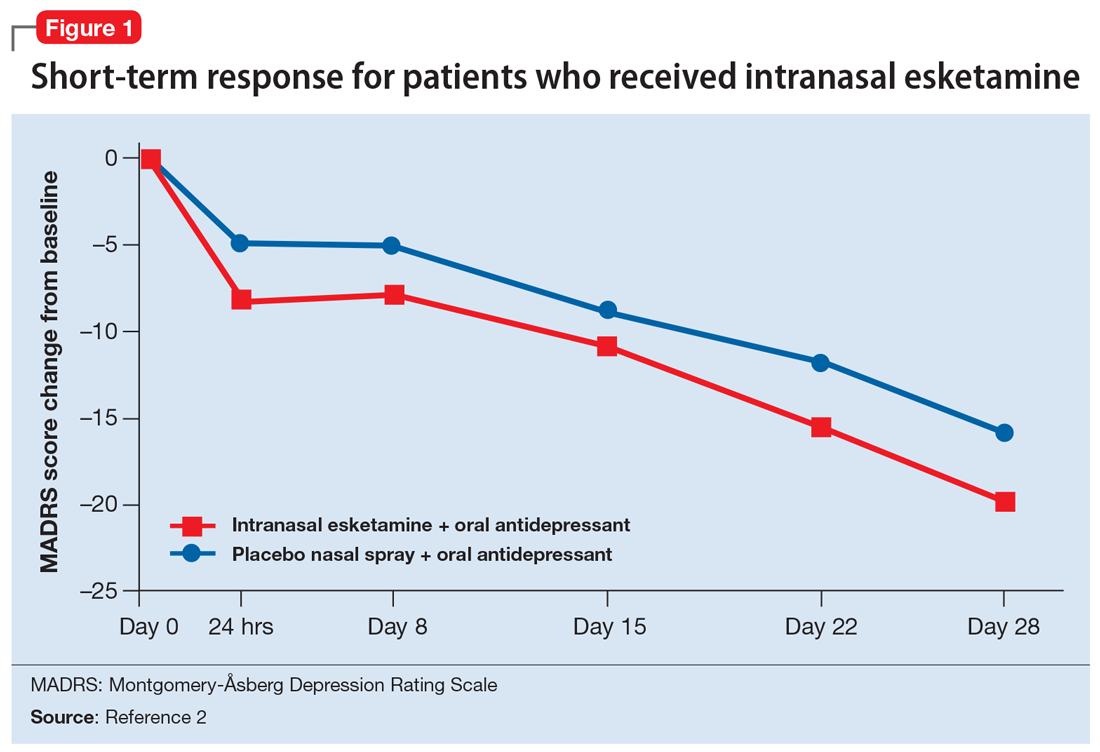
Newly started antidepressants included escitalopram, sertraline, duloxetine, or extended-release venlafaxine. Esketamine intranasal spray was initiated at 56 mg and could be titrated up to 84 mg at the second dose, based on investigator discretion. The mean age was 47; 62% of the patients were female, 93% were White, and 5% were black. The newly initiated oral antidepressant was a selective serotonin reuptake inhibitor in 32% of patients and an serotonin-norepinephrine reuptake inhibitor in 68% of patients. The time course of response for this 4-week, short-term treatment study is illustrated in Figure 1.2 While the primary efficacy measure was improvement of MADRS score at Week 4, the majority of the placebo-active drug separation occurred 24 hours after the initial 56 mg dose of esketamine. Between 24 hours and Day 28, intranasal esketamine showed continued separation from antidepressant plus placebo nasal spray. Investigators could increase both placebo nasal spray or esketamine, with 67% of patients receiving 84 mg twice weekly at Day 28.
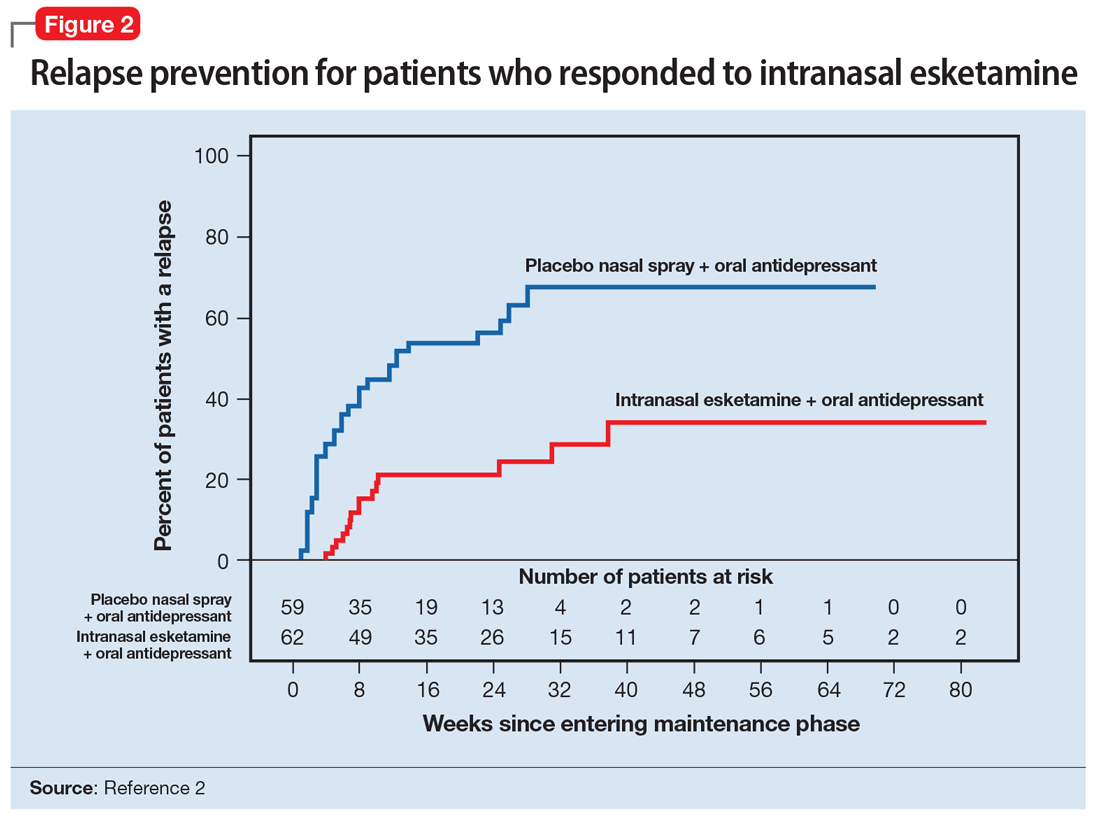
A long-term, double-blind multicenter maintenance-of-effect trial examined adults age 18 to 65 with TRD.5-6 Patients in this study were responders in 1 of 2 short-term studies or in an open-label direct enrollment study. Stable remission was defined as a MADRS total score <12 for at least 3 of the last 4 weeks of the study, and stable response was defined as a MADRS reduction of >50% but not in remission. After 16 weeks of intranasal esketamine plus an oral antidepressant, stable remitters and stable responders were then randomized separately to continue intranasal esketamine or switch to placebo nasal spray, with both groups continuing on their concomitant oral antidepressant. The primary study endpoint was time to relapse. Relapse was defined as a MADRS total score >22 for more than 2 consecutive weeks, hospitalization for worsening of depression, or any other clinically relevant event. The median age was 48, 66% were female, 90% were White and 4% were black. Patients in stable response or stable remission experienced a significantly longer time to relapse compared with patients who continued their oral antidepressant but were switched to placebo intranasal spray. In this remission response study, patients could receive intranasal treatment weekly or bi-weekly based on symptom severity (Figure 22).
Impact on driving. Two studies examined the impact of esketamine on driving performance. One examined adults with major depressive disorder and the other examined healthy participants. The effects of a single 84-mg dose of esketamine nasal spray on a patient’s ability to drive was assessed in 23 healthy adults. In this study, mirtazapine was used as an active control. Driving performance was assessed at 8 hours after treatment with esketamine nasal spray or mirtazapine. Driving performance 8 hours after esketamine nasal spray was similar to placebo and active control. Two participants discontinued the driving task after receiving esketamine due to post-dose adverse reactions. One reported pressure behind the eyes and paresthesia of the hands and feet. The other reported headache and light sensitivity with anxiety.
A second study evaluated the effects of repeated esketamine administration on driving performance in 25 adults with major depressive disorder. In this study, an ethanol-containing beverage was used as an active control. After administration of a single 84-mg dose of intranasal esketamine, driving performance was the same as a placebo at 18 hours. In the multiple dose phase, standard driving performance was similar for esketamine nasal spray and placebo at 6 hours postdose on Days 11, 18, and 25.
Continue to: Pharmacologic profile