These issues are expected to be resolved at the completion of the Study of Tamoxifen and Raloxifene (STAR) trial. This randomized, double-blind comparison of tamoxifen and raloxifene is the largest breast-cancer prevention study ever conducted, involving more than 300 institutions throughout the U.S., Canada, and Puerto Rico and approximately 22,000 postmenopausal women. Participants include women 35 to 59 years of age who face an increased risk of breast cancer and women 60 and older with no additional risk factors. These women are given either 20 mg tamoxifen daily or 60 mg raloxifene daily for 5 years. The study, which was launched in 1999 and is expected to continue for 5 to 10 years, should provide definitive data regarding the role of these SERMs in the prevention of breast cancer and in preventing disease in general in postmenopausal women.
Adverse effects. Although raloxifene exerts several desirable estrogen-agonist and -antagonist effects, it also exerts several undesirable effects (Table 2), which may preclude its administration in a large segment of postmenopausal women. Perhaps its most worrisome side effect is the increased risk of venous thromboembolic events, which has been reported to be approximately 3 times the risk incurred by women on placebo.20,22 (This level of risk is similar to that reported for estrogen use.21) Raloxifene does not improve vasomotor symptoms or vaginal dryness. Leg cramps have been reported in 6% of women on raloxifene versus 2% of women on placebo.23
Raloxifene’s effects on the central nervous system (CNS) are largely unknown and seem to differ from those of estrogen. In an in vitro system using cultured rat neurons derived from areas of the brain important for memory, raloxifene was neuroprotective at low concentrations but neurotoxic at high concentrations. Other animal studies have reported that raloxifene, like estrogen, may have a beneficial effect on cholinergic transmission within the brain and induce neurite outgrowth in ER-positive rat neuronal cell lines.12,24 Because it does not decrease the incidence of hot flushes, some experts believe that raloxifene may exert anti-estrogenic effects on the CNS. However there are few clinical studies of the effects of raloxifene on the CNS in women. The few that exist indicate neither beneficial nor adverse effectson cognition, mood, or memory.25 No clinical data on raloxifene and the risk of Alzheimer’s disease are currently available.
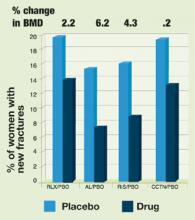
FIGURE 2Effect of bone therapy on vertebral fracture rates
RLX = raloxifene, AL = alendronate, RIS = risedronate, CCTN = calcitonin, PBO = placeboTABLE 1
Effects of raloxifene and ERT on markers of cardiovascular disease risk in healthy postmenopausal women
| Marker | Raloxifene | ERT |
|---|---|---|
| LDL cholesterol | -12 | -14 |
| HDL cholesterol | 0 | +10 |
| HDL2 | +15 | +33 |
| Triglycerides | -4 | +20 |
| Lipoprotein(a) | -7 | -19 |
| Fibrinogen | -10 | -1 |
| Homocysteine | -8 | -6.6 |
| C-reactive protein | -4 | +84.1 |
| Data are reported as percent change compared with placebo except for homocysteine and C-reactive protein, which are reported as percent change from baseline. | ||
| Source: Walsh B, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445-1451. Walsh B, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2000;85:214-218. | ||
TABLE 2
Adverse events reported by postmenopausal women in controlled trials of raloxifene
| Adverse event | Raloxifene | Placebo | P value |
|---|---|---|---|
| Vasomotor symptoms | 9.7% | 6.4% | .01 |
| Leg cramps | 7% | 3.7% | <.001 |
| Influenza syndrome | 13.4% | 11.4% | <.001 |
| Peripheral edema | 5.2% | 4.4% | <.01 |
| Endometrial cavity fluid | 8.1% | 5.7% | .02 |
| Venous thromboembolism | 1% | 0.3% | <.001 |
| Source: Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-644. Nilsen J, et al. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause. 1998;5:211-216. | |||
Patient selection
The candidates for tamoxifen are clearly defined. These include ER-positive breast-cancer patients as well as pre- and postmenopausal women at significant risk for the disease. There currently is no other indication for tamoxifen therapy in the U.S. In other countries, the drug is used—as clomiphene citrate is in the U.S.—to induce ovulation. Because of the risk of venous thromboem-bolism, it is important to limit the use of tamoxifen for the prevention of breast cancer to women at significant risk for the disease.
The best candidates for raloxifene are not as easily defined because several other therapeutic options are available for the same indications. For example, for the prevention and treatment of osteoporosis, options include the bisphosphonates (alendronate and risedronate), calcitonin, ERT, and raloxifene. However, only ERT and raloxifene have other beneficial effects that may be important to postmenopausal women (Table 3). Consequently, to prevent or treat osteoporosis in a postmenopausal woman with vasomotor symptoms and/or urogenital atrophic changes, the best option appears to be ERT, which not only protects against bone loss but relieves menopausal symptoms as well. A similar woman without menopausal symptoms who is concerned about or at risk for breast cancer might best be treated with raloxifene, which not only confers bone protection but may diminish breast cancer risk. However, if the same woman has a history of venous thromboembolism, the bisphosphonates or calcitonin might be better options, since both ERT and raloxifene would expose her to an unnecessarily high risk of a thromboembolic event.