In ER-positive tumors, tamoxifen is believed to exert its anti-tumor effects by inhibiting the estrogen-dependent secretion of growth factors and angiogenic factors by the tumor cells, and by inducing programmed death of the tumor cells.9 An understanding of this mechanism, along with the observation that tamoxifen-treated breast cancer patients not only experience a reduction in breast cancer recurrence but also a 47% reduction in contralateral breast cancer, led to the launch of the tamoxifen chemoprevention trials in North America and Europe.
In 1992, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Tamoxifen Breast Cancer Prevention Trial was launched in the United States and Canada. The results of this trial were released early because of the compelling evidence of tamoxifen’s therapeutic efficacy. The drug was found to reduce the incidence of breast cancer by 44%, 51%, and 55% in women aged 35 to 49, 50 to 59, and 60 or older, respectively. Tamoxifen also was found to reduce ductal carcinoma in situ by 50%. Although tamoxifen decreased the overall occurrence of ER-positive tumors by 69%, it did not have a significant impact on the incidence of ER-negative tumors.11
Based on these results, the FDA approved the use of tamoxifen for the primary prevention of breast cancer in high-risk women. It is important to limit the use of tamoxifen to high-risk women because of the potential for serious side effects, which include endometrial cancer, pulmonary embolism, deep vein thrombosis (DVT), and cataract formation.9-11
The issue of whether tamoxifen inhibits the initial development of a tumor or suppresses an occult tumor remains unresolved. It is possible that both mechanisms are involved. This is not merely an academic concern. Questions remain as to whether a suppressed tumor may develop resistance to tamoxifen or even be stimulated by the drug, becoming more virulent during or after discontinuation of therapy. However, given the extensive data and longterm clinical experience and follow-up with tamoxifen, this scenario seems unlikely. In fact, the reduction in the incidence of breast cancer appears to continue for years after the therapy is discontinued.12,13
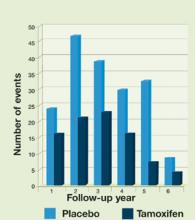
FIGURE 1Effect of tamoxifen on ER-positive breast cancer
Adapted from: Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
Raloxifene
Raloxifene hydrochloride (Evista; Eli Lilly and Company, Indianapolis, Ind) was originally investigated for the treatment of breast cancer and was found to be similar to tamoxifen in its anti-tumor activity. Raloxifene was subsequently studied for its skeletal effects and was approved for osteoporosis prevention in postmenopausal women in December 1997, and for fracture prevention in 1999.
The agent mimics the effects of estrogen on the skeleton and lipids. However, it acts as a complete estrogen antagonist in the breast and the uterus, making it a more desirable SERM in the management of menopausal women with an intact uterus.
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial was a randomized, placebo-controlled, multicenter study involving 7,705 postmenopausal women with osteoporosis at baseline. Although bone metabolism and fractures were the primary endpoints, many other outcomes were evaluated, including uterine and endometrial effects, bleeding, breast cancer incidence, lipid levels, clotting factors, patient tolerance, and adverse events.14-18 The results of these studies support the classification of raloxifene as a SERM with several desirable estrogen-agonistic and -antagonistic effects. Even so, it is far from perfect.
Effects on bone. In the MORE trial, both placebo- and raloxifene-treated postmenopausal women received 500 mg of supplemental calcium and 400 to 600 IU of cholecalciferol (vitamin D) daily. In these postmenopausal women with osteoporosis, a daily dose of 60 mg raloxifene increased bone mineral density (BMD) in the spine by 2.6% and in the femoral neck by 2.1% compared with placebo. The assessment of biochemical bone markers indicated that raloxifene increases BMD by decreasing bone turnover. Indeed, markers of bone resorption and formation—urinary type I collagen Ctelopeptide and serum osteocalcin—were significantly decreased with raloxifene within 3 months of therapy, similar to estrogen.14,19
Raloxifene also reduced vertebral fractures in postmenopausal women with osteoporosis. Vertebral fractures occurred in 10.1% of women randomized to placebo versus 6.6% of women receiving 60 mg raloxifene daily. Although raloxifene decreased the risk of new vertebral fractures regardless of whether a spinal fracture was present at baseline, the absolute percentage decrease in the fracture rate was higher in women who did not have a fracture at baseline.14 This finding underscores the importance of diagnosing and treating osteoporosis before the development of a fracture.
One interesting observation, which is true for raloxifene and other antiresorptive agents, particularly calcitonin, is that the magnitude of the reduction in vertebral fractures is greater than would have been predicted by the modest improvement in BMD observed during therapy. This suggests that antiresorptive agents may influence bone quality, perhaps through its architectural structure, in ways that cannot be ascertained by the assessment of BMD alone. Bone quality refers to skeletal factors that strongly impact the structural properties of bone, independent of the quantity of bone, assessed by BMD. Specific factors that contribute to the structural competence of bone include its microarchitecture, degree of bone mineralization, state of the organic matrix, and rate of bone turnover. The effects of antiresorptive agents on these factors may explain why such modest improvements in BMD—i.e., 2% to 6%—result in reductions in fracture risks in the range of 30% to 50%.