Primary and Secondary Goals
The primary goal of the study was to assess the impact of the HT program on drug interventions, specifically initiating and titrating HFrEF pharmacotherapies. Interventions were based on GDMT with known mortality- and morbidity-reducing properties when used at their maximum tolerated doses, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor-neprilysin inhibitor (ARNi), or angiotensin receptor blockers (ARB), with a preference for ARNi, β-blockers for HFrEF (metoprolol succinate, bisoprolol, or carvedilol), aldosterone antagonists, and SGLT2is.
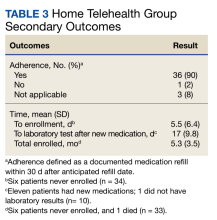
Secondary goals included HF-related hospitalizations, medication adherence, time to enrollment in HT, time to laboratory analysis after the initiation or titration of an ACEi/ARB/ARNi or aldosterone antagonist, and time enrolled in the HT program. Patients were considered adherent if their drug refill history showed consistent fills of their medications. The χ2 test was used for total interventions made during the study period and Fisher exact test for all others.
Results
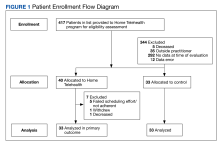
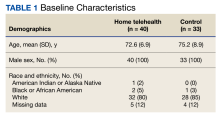
Patient data were collected between July 2022 and June 2023. All 73 patients were male, and the mean age in the HT group (n = 40) was 72.6 years and 75.2 years for the control group (n = 33). Overall, the baseline demographics were similar between the groups (Table 1). Of 40 patients screened for enrollment in the HT program, 33 were included in the analysis (Figure 1).
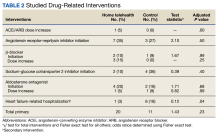
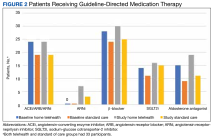
At baseline, the HT group included more individuals than the control group on ACEi/ARB/ARNi (24 vs 19, respectively), β-blocker (28 vs 24, respectively), SGLT2i (14 vs 11, respectively), and aldosterone antagonist (15 vs 9, respectively) (Figure 2). There were 20 interventions made in the HT group compared with 11 therapy changes in the control arm during the study (odds ratio, 1.43; P = .23) (Table 2). In the HT group, 1 patient achieved an ACEi target dose, 3 patients achieved a β-blocker target dose, and 7 achieved a target dose of spironolactone (titration is not required for SGLT2i therapy and is counted as target dose). In the HT group, 17 patients were on ≥ 3 recommended agents, while 9 patients were taking 4 agents. Seven of 20 HT group interventions resulted in titration to the target dose. In the control group, no patients achieved an ARNi target dose, 3 patients achieved a β-blocker target dose, and 2 patients achieved a spironolactone antagonist target dose. In the control arm, 7 patients were on ≥ 3 GDMTs, and 2 were taking 4 agents. No patient in either group achieved a target dose of 4 agents. Five of 11 control group interventions resulted in initiation or titration of GDMT to the target dose.
Of the 40 HT group patients, 7 were excluded from analysis (3 failed to schedule HT, 1 was at a long-term care facility, 1 was nonadherent, 1 declined participation, and 1 died) and 33 remained in the program for a mean (SD) 5.3 (3.5) months. Death rates were tracked during the study: 1 patient died in the HT group and 3 in the control group.
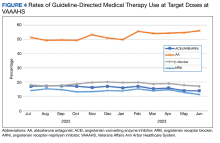
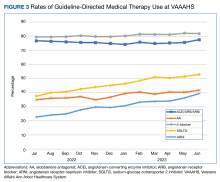
We analyzed the overall percentage of VAAAHS patients with HFrEF who were on appropriate GDMT. Given the ongoing drive to improve HF-related outcomes, HT interventions could not be compared to a static population, so the HT and control patients were compared with the rates of GDMT at VAAAHS, which was available in the Academic Detailing Service Heart Failure Dashboard (Figure 3). Titration and optimization rates were also compared (Figure 4). From July 2022 to June 2023, ARNi use increased by 16.6%, aldosterone antagonist by 6.8%, and β-blockers by 2.4%. Target doses of GDMTs were more difficult to achieve in the hospital system. There was an increase in aldosterone antagonist target dose achievement by 4.7%, but overall there were decreases in target doses in other GDMTs: ACEi/ARB/ARNi target dose use decreased by 3.2%, ARNi target dose use decreased by 2.7% and target β-blocker use decreased by 0.9%.