Methods
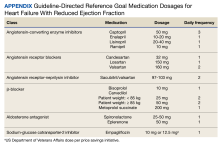
This was a single-center retrospective study of Computerized Patient Record System (CPRS)data. Patients at the VAAAHS were evaluated if they were diagnosed with HFrEF and were eligible for enrollment in the HT monitoring program. Eligibility criteria included a diagnosis of stage C HF, irrespective of EF, and a history of any HF-related hospitalization. We focused on patients with HFrEF due to stronger guideline-based recommendations for certain pharmacotherapies as compared with HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). Initial patient data for HT enrolling were accessed using the Heart Failure Dashboard via the US Department of Veterans Affairs (VA) Academic Detailing Service. The target daily doses of typical agents used in HFrEF GDMT are listed in the Appendix.
The HT program is an embedded model in which HT nurses receive remote data from the patient and triage that with the VAAAHS cardiology team. Patients’ questions, concerns, and/or vital signs are recovered remotely. In this model, nurses are embedded in the cardiology team, working with the cardiologists, cardiology clinical pharmacist, and/or cardiology nurse practitioners to make medication interventions. Data are recorded with an HT device, including weight, blood pressure (BP), heart rate, and pulse oximetry. HT nurses are also available to the patient via phone or video. The program uses a 180-day disease management protocol for HF via remote device, enabling the patient to answer questions and receive education on their disease daily. Responses to questions and data are then reviewed by an HT nurse remotely during business hours and triaged as appropriate with the cardiology team. Data can be communicated to the cardiology team via the patient record, eliminating the need for the cardiology team to use the proprietary portal affiliated with the HT device.
Study Sample
Patient information was obtained from a list of 417 patients eligible for enrollment in the HT program; the list was sent to the HT program for review and enrollment. Patient data were extracted from the VAAAHS HF Dashboard and included all patients with HFrEF and available data on the platform. The sample for the retrospective chart review included 40 adults who had HFrEF, defined as a left ventricular EF (LVEF) of ≤ 40% as evidenced by a transthoracic echocardiogram or cardiac magnetic resonance imaging. These patients were contacted and agreed to enroll in the HT monitoring program. The HT program population was compared against a control group of 33 patients who were ineligible for the HT program. Patients were deemed ineligible for HT if they resided in a nursing home, lacked a VAAAHS primary care clinician, or declined participation in the HT program.
Procedures
Patients enrolled in the HT program were assigned a nurse who monitored and responded to changes in vital signs, such as an increase in weight of ≥ 3 lb in 24 hours or ≥ 5 lb in 7 days. Each participant in HT received a home BP machine to check BP and heart rates and a scale for daily weights. The patient also responded to questions on a tablet device with the BP machine and scale. The vital signs were put into the tablet device, and the patient answered questions about their symptoms for that day. If the patient endorsed any symptoms of HF, such as pedal edema, abdominal distention, orthopnea, paroxysmal nocturnal dyspnea, dyspnea on exertion, or orthopnea, they received a call from the nurse to discuss symptoms and make appropriate adjustments in the diuretic or HF regimen. The HF-trained pharmacist served as a resource for drug therapy management and provided recommendations for diuretic adjustments on an as-needed basis.
Patients who declined participation in the HT program followed the standard of care, which was limited to visits with primary care clinicians and/or cardiologists as per the follow-up plan. Patient data were collected over 12 months. The study was approved by the VAAAHS Institutional Review Board (reference number, 1703034), Research and Development Committee, and Research Administration.