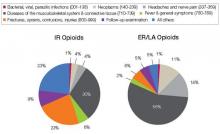
And what are you prescribing them for? Data from a 2009 survey of the prescribing habits of 3200 office-based physicians in 30 specialties showed that most prescriptions written for ER/LA and IR opioids are associated with diagnoses related to pain in the musculoskeletal system and connective tissue (56% [ER/LA] and 30% [IR]). For ER/LA
prescriptions the second most common diagnoses were headaches and nerve pain (14%), while for IR prescriptions they were fractures, sprains, and contusions (23%) [Figure 4].3
| FIGURE 4: Diagnoses associated with use (by grouped ICD-9 codes) for IR and ER/LA opioids as reported by office-based physicians in the United States, 2009 |
| ER, extended release; IR, immediate release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugs AdvisoryCommittee/UCM220950.pdf. |
According to Janet Woodcock, MD, Director of the FDA’s Center for Drug Evaluation and Research, some physicians may not be clear about who should receive these drugs or how to manage patients in pain. As a result, some physicians may be reluctant to prescribe opioid analgesics, leaving patients without adequate pain relief. At the same time, other physicians overprescribe them, putting patients—and anyone with access to the family medicine cabinet—at risk.4
A REMS by any other name
And so REMS was conceived. On February 6, 2009, manufacturers of certain opioid drug products received a letter from the FDA informing them that their drugs would be required to have a risk management program, and inviting them to meet to discuss the design and development of such a REMS.5
Two years later, on April 19, 2011, an alarm in the form of an action plan was released by the Obama administration through the Office of National Drug Control Policy. The plan,
Epidemic: Responding to America’s Prescription Drug Abuse Crisis, outlined a set of measures to remedy the problem through education, monitoring, proper disposal of prescription drugs, and enforcement.6
REMS for opioids was the FDA’s response in support of the President’s plan. On the same day in April, 32 manufacturers of ER/LA opioids received a letter from the FDA informing them that they must meet new safety requirements concerning these medications under a single shared, standardized system [Table].
| TABLE: Long-acting and extended-release opioids requiring an opioid REMS |
| Brand Name Products |
| Trade Name | Generic Name | Sponsor | |
| 1 | Duragesic | Fentanyl transdermal system | Ortho-McNeil-Janssen |
| 2 | Dolophine | Methadone HCI tablets | Roxanne Laboratories |
| 3 | Avinza | Morphine sulfate extended-release capsules | King Pharmaceuticals/Pfizer |
| 4 | Kadian capsules | Morphine sulfate extended-release capsules | Actavis |
| 5 | MS Contin | Morphine sulfate controlled-release tablets | Purdue Pharma |
| 6 | Oramorph | Morphine sulfate sustained-release tablets | Xanodyne Pharmaceuticals |
| 7 | OxyContin | Oxycodone HCI controlled-release tablets | Purdue Pharma |
| 8 | Opana ER | Oxymorphone HCI extended-release tablets | Endo Pharmaceuticals |
| 9 | Exalgo | Hydromorphone HCI extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 10 | Butrans | Buprenorphine transdermal system | Purdue Pharma |
| Generic Products |
| Drug Name | Generic Name | Sponsor | |
| 1 | Fentanyl | Fentanyl extended-release transdermal system | Actavis |
| 2 | Fentanyl | Fentanyl extended-release transdermal system | Lavipharm Labs |
| 3 | Fentanyl | Fentanyl extended-release transdermal system | Mallinckrodt Inc/Covidien |
| 4 | Fentanyl | Fentanyl extended-release transdermal system | Mylan Technologies |
| 5 | Fentanyl | Fentanyl extended-release transdermal system | Noven Pharmaceuticals |
| 6 | Fentanyl | Fentanyl extended-release transdermal system | Teva Pharmaceutical Industries |
| 7 | Fentanyl | Fentanyl extended-release transdermal system | Watson Pharmaceuticals |
| 8 | Methadone hydrochloride | Methadone HCl oral solution | The Pharmanetwork |
| 9 | Methadone hydrochloride | Methadone HCl oral solution | Mallinckrodt Inc/Covidien |
| 10 | Methadone hydrochloride | Methadone HCl oral solution | Sandoz |
| 11 | Methadone hydrochloride | Methadone HCl oral solution | Roxane Laboratories |
| 12 | Methadone hydrochloride | Methadone HCl oral solution | VistaPharm |
| 13 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Endo Pharmaceuticals |
| 14 | Morphine sulfate | Morphine sulfate extendedrelease tablets | KV Pharmaceuticals |
| 15 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 16 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Watson Pharmaceuticals |
| 17 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Rhodes Pharmaceuticals |
| 18 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 19 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Impax Laboratories |
| 20 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Teva Pharmaceutical Industries |
| 21 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Endo Pharmaceuticals |
| 22 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Impax Laboratories |
| 23 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Actavis |
| *Tentatively approved products. Source: U.S. Food & Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafet/InformationbyDrugClass/ucm251735.htm. |
As outlined in this REMS, manufacturers must provide for the training of prescribers of opioid medications—training that covers proper patient selection, patient counseling in specific product use and risk, and assessment for addiction and tolerance. Manufacturers must also develop factual, nonpromotional patient information and medication guides that will be FDA regulated and approved. Finally, they will be asked to adhere to a timetable to assess whether REMS is meetings its goals.4,5
In May 2011, the FDA met with manufacturers to expand on how to coordinate and implement the REMS requirements.
Hope for a “new normal”
Will REMS for other large medication classes eventually reach beyond opioid analgesics, perhaps warranting practitioners to view REMS as being a good thing as opposed to a nuisance? Your decision to participate in REMS or pass and alter your care approach will need to be made soon. What will you do?
For you as an opioid prescriber, education is the focus, and you will soon be presented with voluntary prescriber education programs. The “hope” is that you will volunteer to take the opioid education program, fill out an electronic or fax form, and send it in to an administrator who will track all those who participate. Since “hope” will unlikely drive large-scale participation, when hope finally runs out the education will become mandatory. This will occur in a year or 2, and will likely become a Drug Enforcement Administration requirement for you to procure CII scheduling.