Are you aware that a significant change is coming to the way you prescribe opioid pain relievers for your patients? After 3 years of debate among the Food and Drug Administration (FDA), drug industry stakeholders, members of the pain and addiction communities, patient advocacy groups, and the public, the first large-scale, class-wide REMS is here. REMS is the acronym for Risk Evaluation and Mitigation Strategies. There is a good chance you are prescribing one or more of the affected medications, and adherence to the REMS requirements will be essential if you wish to continue prescribing them.
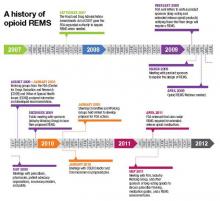
Before getting into the fine points of the opioid REMS, a little background about how it came into being is in order. On March 25, 2008, the Food and Drug Administration Amendments Act went into effect, granting the FDA authority to require a REMS for any product or product class it deemed to be a public health, safety, or welfare threat. Basically, REMS is an FDA-imposed “safety” program. The first medication to now have a single or class REMS is the class of extended-release (ER) and long-acting (LA) opioid analgesics.
Why opioid analgesics? In 2007, attempts to mitigate targeted risks associated with 30 drugs using RISKMaps were cited as inadequate by the FDA. RISKMaps are safety programs designed to minimize significant risks of certain medicines through FDA-approved labeling, reporting of adverse events, prescriber and patient education about risks, reminders, and performance-linked access systems that tie access to medications with documentation and laboratory testing.1 Passage of the FDA Amendments Act allowed the FDA to use its REMS authority to “improve” existing risk plans.
Forces for change
The FDA cites many good reasons for this change, primarily to ensure that the benefits of prescribing opioid analgesics outweigh the risks, and that patients in pain who need these drugs have access to them. Driving factors behind this move centered on the highly visible consequences associated with what FDA experts describe as misuse, abuse, and improper prescribing of 12 ER/LA opioid analgesics. According to FDA estimates, in 2007 more than 33 million Americans age 12 and older misused ER/LA opioids. Of the almost 28,000 Americans who died from unintended consequences of drug use, nearly 12,000 were associated with prescription analgesics.2
In my opinion, voluntary continuing medical education (CME) and professional organization guidelines added to the problem by failing to decrease overdoses and unintended deaths. This may come as no surprise, as such deaths often stem from diversion, and diverters typically are not subject to a CME requirement.
The ER/LA segment of the class was targeted for a variety of reasons. First, higher doses of ER/LA opiates packed into single units are believed to pose a greater threat than the millions of short-acting, immediate-release (IR) opioid analgesics units abused annually.3 Another reason for the move focused on the burden to the health system caused by more than 24 similar individual REMS existing in this class. That alone created a virtual paper, regulatory, and health system encumbrance that is expected to be alleviated by a class-wide REMS.
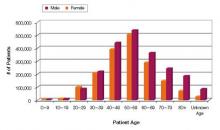
Increasing numbers of prescriptions were an additional consideration. The number of outpatient retail prescriptions dispensed for ER/LA and IR opiates rose dramatically between 2000 and 2009, from 9.3 million to 22.9 million ER/LA opioids and from 164.8 million to 234 million IR opioids [Figure 1].3 Who is prescribing them? You are. In 2009, primary care physicians were the top prescribers of ER/LA (43.8%) and IR (42.1%) opioid analgesics [Figure 2].3 Who are you prescribing them for? Not the elderly age group you might expect. The largest number of prescriptions were written for men and women between ages 50and 59 [Figure 3].3
| FIGURE 1: Total number of prescriptions dispensed for ER/LA and IR opioids from US outpatient retail pharmacies, 2000-2009. |
| ER, extended release; IR, immediate release; LA, long acting; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterial/Drug/AnestheticAndLifeSupportDrugsAdvisory Committee/UCM220950.pdf. |
| FIGURE 2: Total number of prescriptions dispensed in the United States by top 10 prescribing specialties for IR and ER/LA opioids, 2009 |
| ANES, anesthesiologists; DO, doctor of osteopathy; EM, emergency medicine; ER, extended release; FM, family medicine; GP, general practitioner; HEM, hematologists; IM, internal medicine; IR, immediate release; LA, long acting; NP, nurse practitioners; ORTH SURG, orthopedic surgeons; NEURO, neurologists; PA, physician assistants; PM&R, physical medicine and rehabilitation; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |
| FIGURE 3: Total number of unique patients, stratified by age and sex, receiving a dispensed prescription for an ER/LA opioid product from US outpatient retail pharmacies, 2009 |
| ER, extended release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |