Management
Current treatments for PN yield varied results. Many patients with moderate to severe PN attempt multiple therapies before seeing improvement.31 Treatments include topical, oral, and injectable medications and are either directed at the neural or immune components of PN due to the interplay between increased nerve fibers in the lesions (neural axis) as well as increases in cytokines and other immunologic mediators (immune axis) of this condition. However, the FDA recently approved the first treatment for PN—dupilumab—which is an injectable IL-4 receptor antagonist directed at the immunologic interactions affiliated with PN.
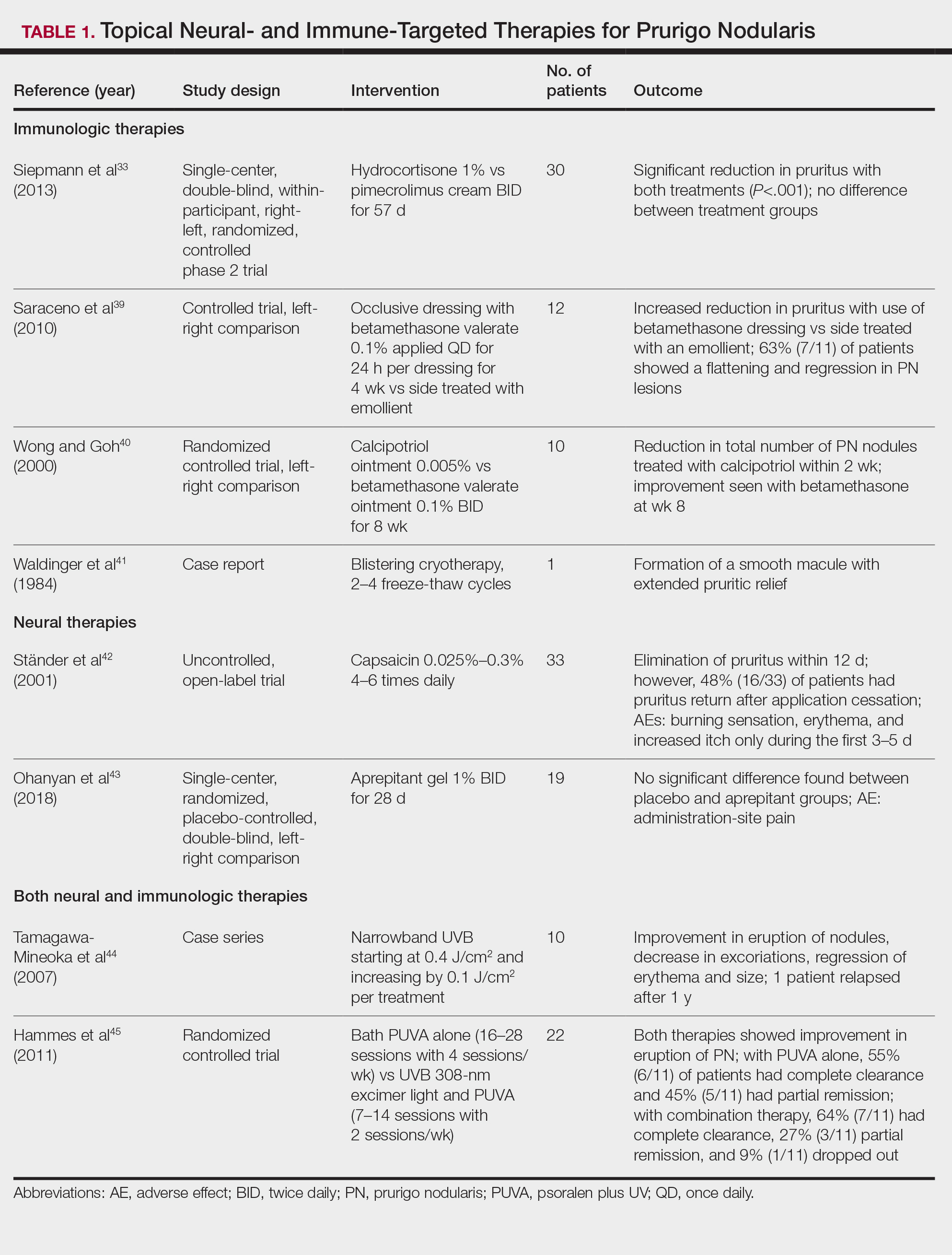
Immune-Mediated Topical Therapies—Immunologic topical therapies include corticosteroids, calcipotriol, and calcineurin inhibitors. Studies that have analyzed these treatments are limited to case reports and small intraindividual and randomized controlled trials (Table 1). Topical therapies usually are first-line agents for most patients. Adverse effects include transient irritation of the skin.40,42,43
Cryotherapy is another topical and immunologic therapy for those with PN; however, this treatment is more appropriate for patients with fewer lesions due to the pain that accompanies lesions treated with liquid nitrogen. In addition, this therapy can cause dyspigmentation of the skin in the treated areas.41
Similar to cryotherapy, intralesional corticosteroid injections are appropriate for patients with few PN lesions. A recent report described intralesional corticosteroid injections of 2.5 mg/mL for a PN nodule with high efficacy.46,47 This treatment has not undergone trials, but success with this modality has been documented, with adverse effects including hyperpigmentation or hypopigmentation in the treated area and transient pain.46
Neural-Mediated Topical Therapies—Neural topical therapies include capsaicin and neurokinin-1 receptor antagonists, aprepitant43 and serlopitant. These treatment studies are limited to small open-label and randomized controlled trials. Adverse effects of these treatments include transient cutaneous pain at the site of topical administration. In addition, neural-mediated topical therapies have shown either limited improvements from baseline or return of symptoms after treatment cessation.42,43
Supplements—N-acetyl cysteine is an over-the-counter supplement that has been reported to improve symptoms in patients with skin-picking disorders.48 The mechanism of action includes antioxidant effects such as decreasing reactive oxygen species, decreasing inflammatory markers, regulating neurotransmitters, and inhibiting hyperkeratosis.49 N-acetyl cysteine has been poorly studied for its application in PN. A small study of 3 patients with subacute PN receiving 1200 mg of oral N-acetyl cysteine reported varying levels of improvement in skin appearance and reduction in skin picking.50
Phototherapy—Phototherapy, a typical first- or second-line treatment modality for PN, targets both the neural- and immune-mediated aspects associated with pruritus in PN (Table 1).51 UV light can penetrate through the epidermal layer of the skin and reach the keratinocytes, which play a role in the immune-related response of PN. In addition, the cutaneous sensory nerves are located in the upper dermal layer, from which nerve fibers grow and penetrate into the epidermis, thereby interacting with the keratinocytes where pruritic signals are transmitted from the periphery up to the brain.51
Studies analyzing the effects of phototherapy on PN are limited to case series and a small randomized controlled trial. However, this trial has shown improvements in pruritus in the participants. Adverse effects include transient burning and erythema at the treated sites.44,45
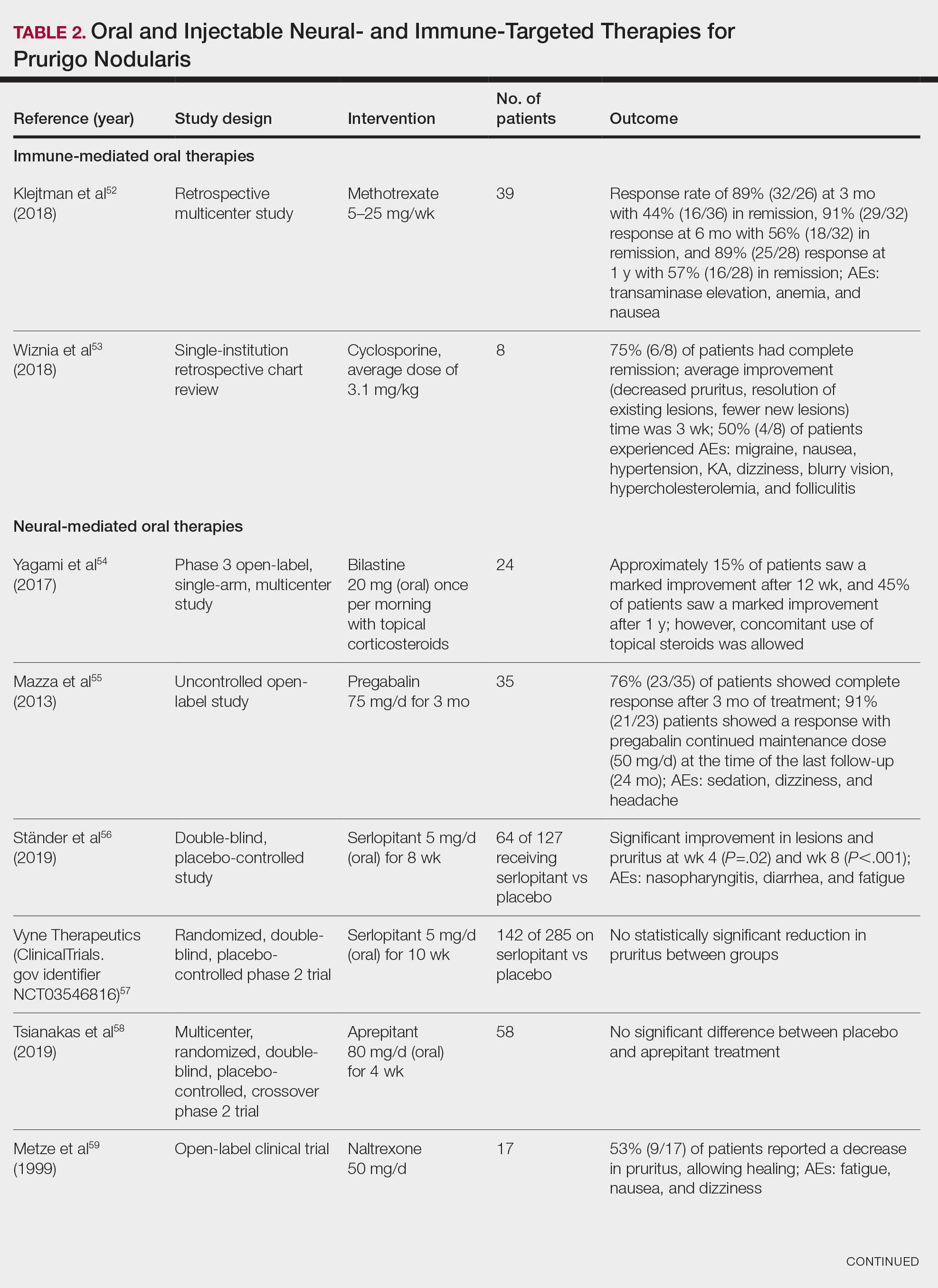
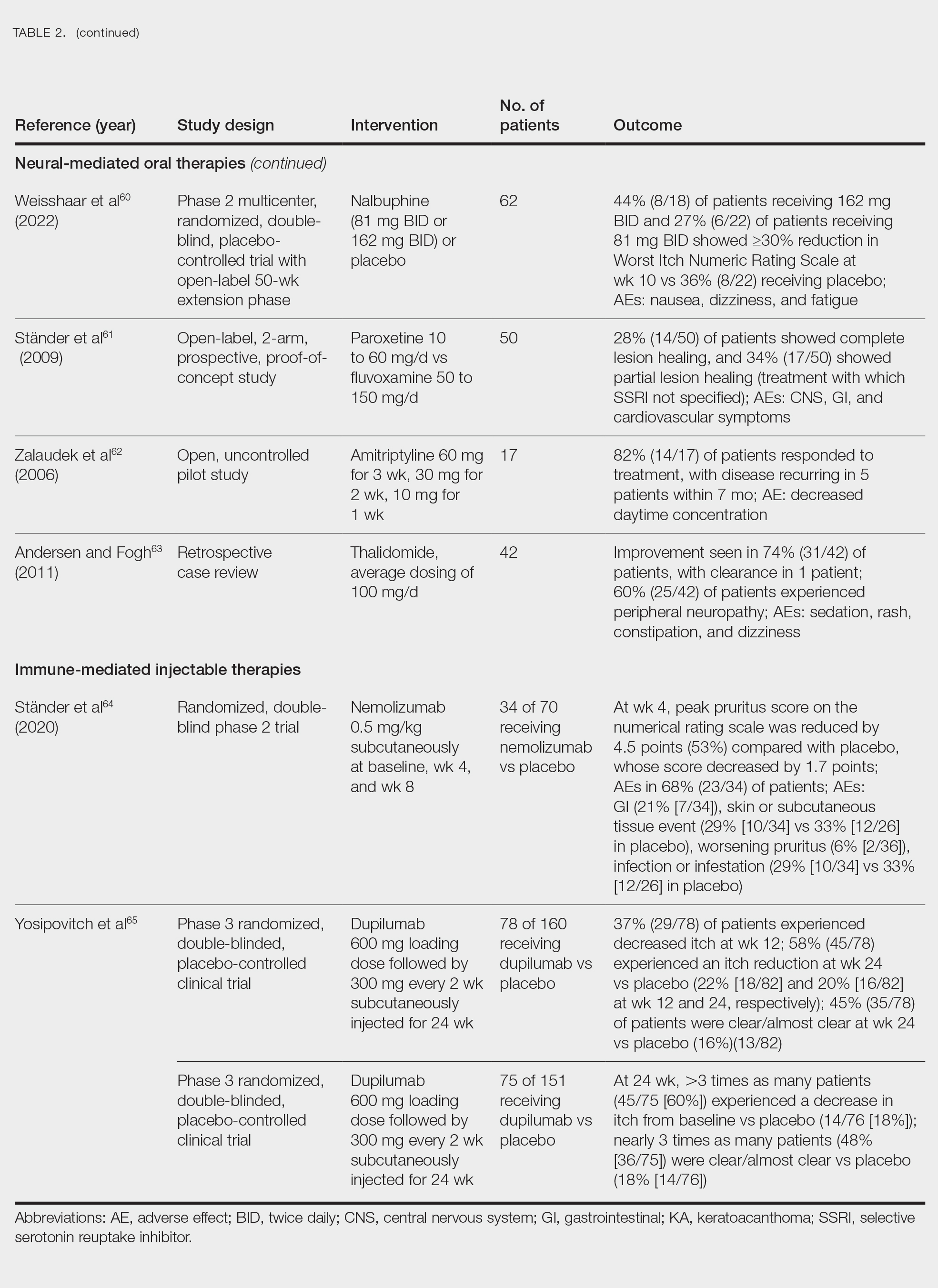
Immune-Mediated Oral Therapies—Immunologic-targeted oral therapies include bilastine, methotrexate, and cyclosporine (Table 2).52,53 Bilastine efficacy was analyzed in a small phase 3, open-label, multicenter study in Japan; however, patients were allowed to use topical steroids in conjunction with the oral antihistamine.54 Methotrexate and cyclosporine are immunosuppressive medications and were analyzed in small retrospective studies. Both treatments yielded notable relief for patients; however, 38.5% (15/39) of patients receiving methotrexate experienced adverse events, and 50.0% (4/8) experienced adverse events with cyclosporine.52,53
Neural-Mediated Oral Therapies—Neural-targeted oral therapies include pregabalin, serlopitant, aprepitant, naltrexone, nalbuphine, SSRIs (paroxetine and fluvoxamine), amitriptyline, and thalidomide. The research on these treatments ranges from case reviews to randomized controlled trials and open-label trials (Table 2).55-63
Thalidomide was studied in a small retrospective case review that showed notable improvement in PN. Dosages of thalidomide varied, but on average the dose was 100 mg/d. However, greater than 50% of patients experienced at least 1 adverse effect with this treatment.63
A study performed in Italy showed promising results for patients treated with pregabalin, with 70.0% (21/30) continuing to take pregabalin for almost 2 years following completion of the initial 3-month trial.55 Naltrexone decreased pruritus in more than half of patients (9/17).59 Amitriptyline yielded improvements in patients with PN; however, disease recurred in 5 patients (29%) after 7 months.62 A study performed in Germany reported promising results for paroxetine and fluvoxamine; however, some patients enrolled in the study had some form of psychiatric disorder.61
Serlopitant, aprepitant, and nalbuphine were studied in randomized controlled trials. The serlopitant trials were the largest of the neurally mediated oral medication studies; one showed substantial improvement in patients with PN,56 while the most recent trial did not show significant improvement (ClinicalTrials.gov identifier NCT03546816).57 On the other hand, aprepitant showed no major difference between the experimental and placebo groups.58 Nalbuphine 162 mg twice daily showed greater improvement in PN than nalbuphine 81 mg twice daily.60
Immune-Mediated Injectable Therapies—Immune-targeted injectables include nemolizumab and dupilumab (Table 2). Nemolizumab is an IL-31 antagonist that has been studied in a small randomized controlled trial that showed great success in decreasing pruritus associated with PN.64 IL-31 has been implicated in PN, and inhibition of the IL-31 receptor has been shown to disrupt the itch-scratch cycle of PN. Dupilumab is a monoclonal antibody against the IL-4 and IL-13 receptors, and it is the only FDA-approved treatment for PN.65 Blockage of these protein receptors decreases type 2 inflammation and chronic pruritus.66,67 Dupilumab is FDA approved for the treatment of atopic dermatitis and recently was approved for adults with PN. Dupilumab acts to block the shared α-subunit of the pruritogenic cytokines IL-4 and IL-13 pathways,29 thereby breaking the itch-scratch cycle associated with PN and allowing for the healing of these lesions. Results from 2 clinical trials showed substantially reduced itch in patients with PN.65 Dupilumab also was approved by the European Medicines Agency for moderate to severe PN.68
Conclusion
Prurigo nodularis is a chronic condition that affects patient quality of life and can mimic various dermatologic conditions. The epidemiology and pathophysiology of PN have not been fully expounded. More research should be conducted to determine the underpinnings of PN to help identify more consistently effective therapies for this complex condition.