News
Drug granted priority review for relapsed/refractory AML
- Author:
- HT Staff
The US Food and Drug Administration (FDA) has granted priority review for the new drug application (NDA) for enasidenib (AG-221), an inhibitor of...
News
Hospital floors pose infection risk, team says
- Author:
- HT Staff
Hospital room floors may be an overlooked source of infection, according to a study published in the American Journal of Infection Control....
News

Antithrombotic drugs may raise risk of subdural hematoma
- Author:
- HT Staff
Use of antithrombotic drugs is associated with a higher risk of subdural hematoma, according to a case-control study of more than 400,000...
News

Hemophilia repository open to US scientists
- Author:
- HT Staff
A new resource has been made available for US-based scientists studying hemophilia A and B. The resource—known as the My Life, Our Future...
News

CCSs’ subsequent cancer risk decreased from ’70s to ’90s
- Author:
- HT Staff
Childhood cancer survivors (CCSs) who were diagnosed in the 1990s have a lower risk of subsequent malignancies than CCSs diagnosed in the 1970s,...
News
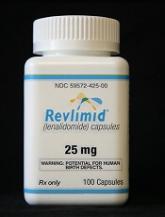
FDA expands approved indication for lenalidomide
- Author:
- HT Staff
The US Food and Drug Administration (FDA) has approved a new indication for lenalidomide (Revlimid). The drug is now approved for use as...
News
VZV vaccine reduces HZ incidence after HSCT
- Author:
- HT Staff
ORLANDO, FL—Results of a phase 3 trial suggest an inactivated varicella zoster virus (VZV) vaccine known as V212 can reduce the risk of herpes...
News

Acquired mutations may compromise assay
- Author:
- HT Staff
A newly discovered issue with the Ba/F3 transformation assay could “jeopardize attempts to characterize the signaling mechanisms and drug...
News

Vaccine can fight different malaria strains
- Author:
- HT Staff
An investigational malaria vaccine can protect healthy adults from infection with a malaria strain different from that contained in the vaccine,...
News

FDA clears push-button blood draw device
- Author:
- HT Staff
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a push-button blood collection device called TAP. It provides “...
News
CHMP recommends authorization of antiemetic agent
- Author:
- HT Staff
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic...
News
CHMP advocates approval of edoxaban product
- Author:
- HT Staff
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that Roteas receive marketing authorization...
News
CHMP recommends new indication for daratumumab
- Author:
- HT Staff
The CHMP recommended approving daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone as treatment for...
News
Docs create guideline to aid workup of acute leukemia
- Author:
- HT Staff
Two physician groups have published an evidence-based guideline that addresses the initial workup of acute leukemia. The guideline includes 27...
News

First case of artemisinin resistance in Africa
- Author:
- HT Staff
Researchers have identified the first known case of artemisinin-resistant malaria originating in Africa, according to a letter published in NEJM...
