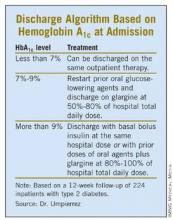
PHILADELPHIA – An algorithm based on hemoglobin A1c levels at admission was safe and effective in guiding diabetes therapy after discharge, based on a prospective, randomized pilot study of 224 inpatients with type 2 diabetes.
Average HbA1c dropped from 8.4% on admission to 7.9% at 4 weeks and to 7.3% at 12 weeks after discharge, said Dr. Guillermo E. Umpierrez, professor of medicine at Emory University, and chief of diabetes and endocrinology at Grady Memorial Hospital, Atlanta.
"If we can do this at Grady Hospital, a county hospital in downtown Atlanta, I think most of you can do it in your facilities. We see these patients within 2 weeks of discharge. I think frequent followup and education are key to [the success of] this type of program," Dr. Umpierrez said.
The patients were placed into one of three post-discharge treatment groups based on their HbA1c levels at admission. Patients with admission HbA1c values of less than 7% resumed their previous outpatient treatments at discharge. Those with HbA1c values of 7%-9% resumed their outpatient oral agents at discharge along with once-daily glargine at 50%-80% of the hospital dose. Patients admitted with a HbA1c level above 9% were discharged on their oral agents plus glargine at 80%-100% of the hospital dose or with the same basal and bolus insulin doses as they’d been given in the hospital.
The treatment goal after discharge was a blood glucose range of 70-130 mg/dL (fasting and premeal) and a HbA1c value below 7%, Dr. Umpierrez said.
For the group discharged on oral agents alone, average HbA1c fell from 6.9% at admission to 6.6% at 12 week follow-up. For those discharged on oral agents plus glargine, average HbA1c fell from 9.2% at admission to 7.5% at 12 weeks. For those discharged on glargine plus glulisine, average HbA1c fell from 11.1% to 8.0% at 12 weeks, Dr. Umpierrez reported.
Hypoglycemia with blood glucose levels below 70 mg/dL occurred post-discharge in 22% of those discharged on oral agents, 30% on oral agents plus glargine, and 44% on glargine plus glulisine. However, hypoglycemia with blood glucose values less than 40 mg/dl occurred in 3% of all patients and in 6% of the group on glargine plus glulisine.
The 224 study patients were a subset of the 375 participants in Sanofi-Aventis’ multicenter "Basal Plus" trial. That study of medical and surgical inpatients with previously diagnosed type 2 diabetes compared the efficacy and safety of a daily dose of glargine plus corrective doses of glulisine ("basal plus") to basal-bolus insulin and sliding scale regular insulin regimens.
At admission, the mean blood glucose level was 204 mg/dL (range 140-400 mg/dL), and the mean admission HbA1c was 8.4%. A total of 150 patients were randomized to the basal-bolus regimen, 148 to "basal plus" regimen, and 77 to the sliding scale insulin regimen.
During hospitalization, patients in the basal-bolus group were started at 0.5 U/kg, given half as glargine once daily and half as glulisine before meals. The "basal plus" group received 0.25 U/kg of glargine once daily plus correction doses of glulisine before meals for blood glucose values above 140 mg/dL. Sliding scale insulin was given four times a day for blood glucose values above 140 mg/dL.
Results from the in-hospital patients in the basal plus trial were reported by Dr. Umpierrez and his fellow researchers in a poster. The basal plus and basal bolus regimens resulted in similar glycemic control, with worse outcomes for those on sliding scale insulin. Mean daily blood glucose values after day 1 were 172 mg/dL for sliding scale insulin, 156 mg/dL for basal bolus, and 163 mg/dL for basal plus. Treatment failures, defined as more than two consecutive blood glucose values or a mean daily glucose value greater than 240 mg/dL, occurred in 19% on sliding scale insulin and in 2% on the basal plus regimen (P less than .001). Glucose values of less than 70 mg/dL occurred in 16% of inpatients and in 1.7% of blood glucose readings overall. Glucose levels of less than 40 mg/dL occurred in 1% of the basal bolus and the basal plus groups, and in none of the inpatients on sliding scale insulin.
There were no differences in length of stay or complications including wound infections, pneumonia, respiratory or renal failure and bacteremia between groups, the investigators reported.
This study was funded by Sanofi-Aventis. Dr. Umpierrez disclosed that he has also received research support from Merck.