Article

Cannabis for chronic pain: Not a simple solution
Real concerns include amotivation, worsening psychiatric states, chronic disability, and chemical dependence.
Vania Modesto-Lowe, MD, MPH
Connecticut Valley Hospital, Middletown, CT; Quinnipiac University, Hamden, CT; University of Connecticut School of Medicine, Farmington
Rachel Bojka, MS, PA-C
Quinnipiac University, Hamden, CT
Camille Alvarado, DO, MPH
University of Connecticut School of Medicine, Farmington
Address: Vania Modesto-Lowe, MD, MPH, Connecticut Valley Hospital, PO Box 351, Silver Street, Middletown, CT 06457; vania.modesto-lowe@ct.gov
Cannabis may be an effective alternative or adjunctive
treatment for peripheral neuropathy, an often debilitating
condition for which standard treatments often provide
little relief. Most studies show moderately improved pain
from inhaled cannabis use, but adverse effects such as
impaired cognition and respiratory problems are common,
especially at high doses. Data on the long-term
safety of cannabis treatments are limited. Until riskbenefi
t profi les are better characterized, doctors in states
where cannabis therapy is legal should recommend it for
peripheral neuropathy only after careful consideration.
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
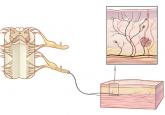
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.

Real concerns include amotivation, worsening psychiatric states, chronic disability, and chemical dependence.
A practical approach for identifying an underlying cause is to first screen for common ones.

