Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
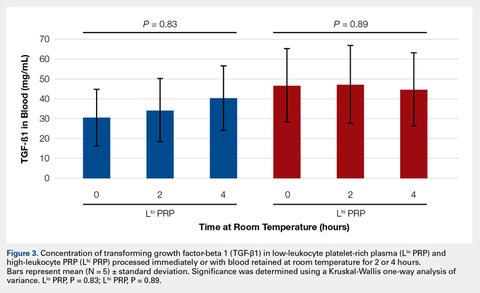
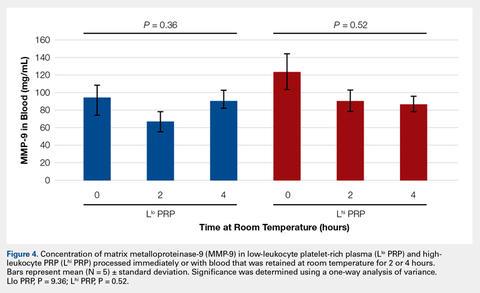
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
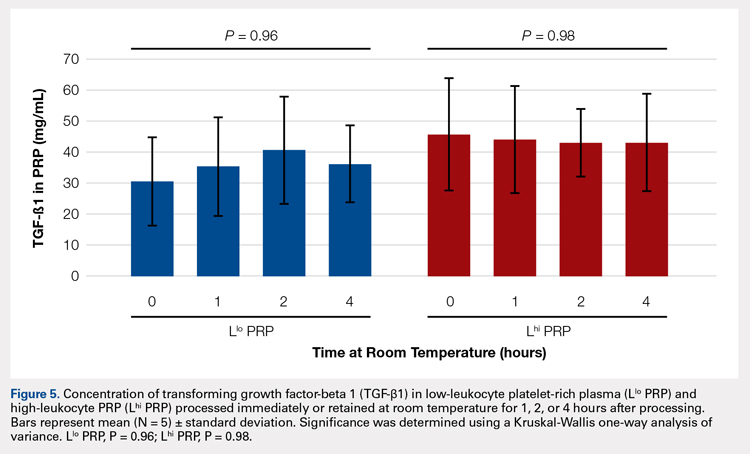
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5).
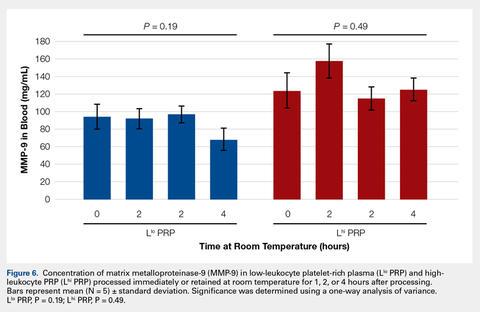
Bioactive/pro-MMP-9 concentrations were also unaffected by retention at room temperature for up to 4 hours (Figure 6).Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| Platelet Concentration (thou/uL) | |||||
Automated Count | Manual Count | P Value | |||
Mean ± SD | Range | Mean ± SD | Range | ||
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...