Total knee arthroplasty (TKA) procedures are projected to increase by more than 6-fold by 2030, with concurrent increases in revision TKA for infection projected.1 Infection after TKA remains one of the most serious complications of the procedure, occurring in <2% of primary TKAs.2 The majority of prosthetic joint infections (PJIs) are caused by staphylococci and streptococci.3 Although infection and treatment of PJIs by mycobacterial species have been described, there are presently no established treatment guidelines for mycobacterial PJIs.4,5
Given the scarcity of clinical experience in dealing with these organisms, and the predicted increasing incidence of revision knee arthroplasty due to infection, we describe an unusual case of a PJI caused by Mycobacterium abscessus (M. abscessus), which was successfully treated using a combination of antimicrobial therapy and staged reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
BACKGROUND
Mycobacteria are common environmental organisms that can survive harsh conditions, including low pH and extreme temperatures. They form biofilms and may be difficult to eradicate in cases of infection.6 M. abscessus has proven to be difficult to eradicate due to limited antimicrobial susceptibility, lack of bactericidal options, and the variable presence of the erm gene, which yields inducible resistance to macrolides.7 Post-procedural outbreaks due to mycobacteria have been reported, often attributed to contaminated multiuse instruments, inadequate sterilization of tap water, multiuse vials, or improper skin preparation.6,8-13
CASE REPORT
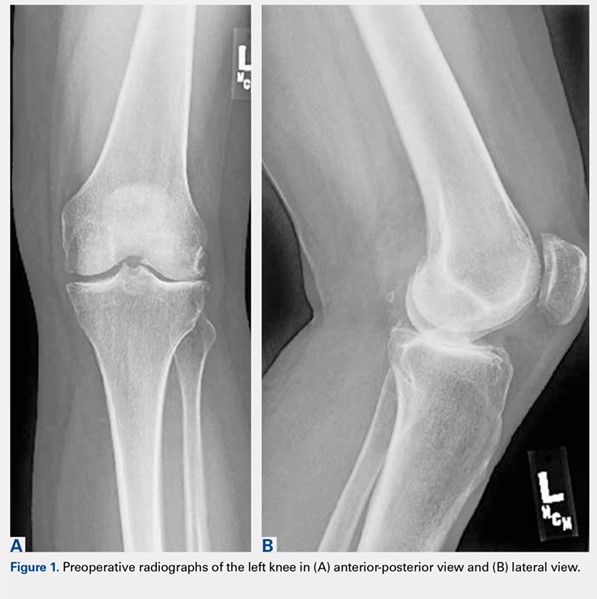
A 61-year-old woman was referred with a 3-year history of progressive left knee pain and swelling. Before 8 months, she had undergone knee arthroscopy and had been treated with multiple steroid and hyaluronic acid injections, as well as ultrasound-guided aspiration of a Baker’s cyst (Figures 1A, 1B).
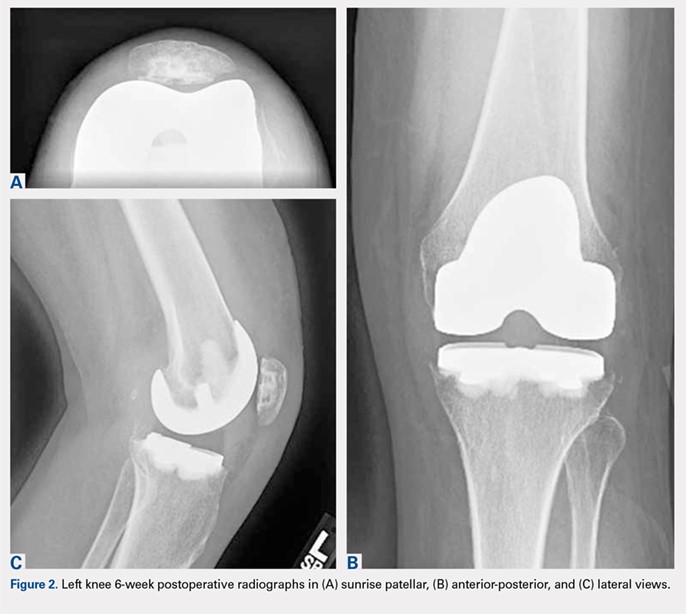
She elected to proceed with TKA 1 month after her last steroid injection. There was no preoperative concern for native joint infection. At the time of arthroplasty, clear joint fluid was encountered, and a deep tissue culture was taken (Figures 2A-2C).
Routine screening cultures for acid-fast bacilli (AFB) returned positive 9 days after the index arthroplasty, with subsequent identification of a nontuberculous mycobacterium (NTM), M. abscessus, subspecies massiliense. Sensitivity tests revealed susceptibility to amikacin, cefoxitin, and tigecycline (Table 1). The isolate was found to have inducible macrolide resistance by erm gene testing.
Table 1. Initial Mycobacterium abscessus massiliense Susceptibilities
Medication | Minimum Inhibitory Concentration |
Amikacin | 16 (S) |
Cefoxitin | 16 (S) |
Imipenem | 8 (I) |
Linezolid | 16 (I) |
Clarithromycin | 2 (S)a |
Tigecycline | 1 (S) |
aAt 3 days; erm gene detected at 7 days.
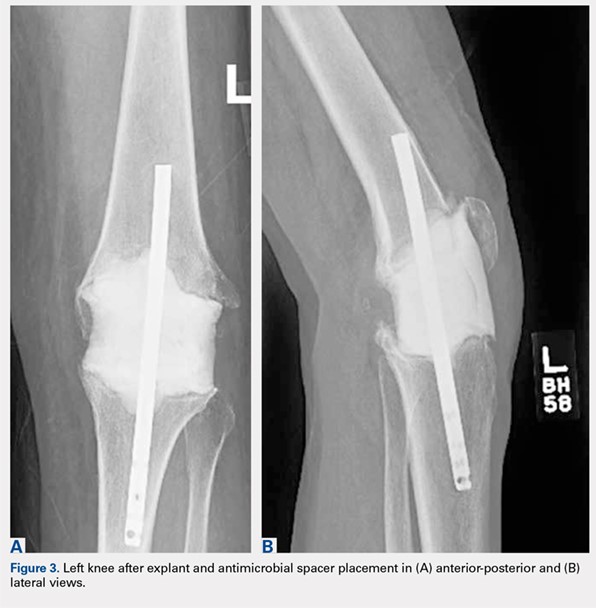
Given no prior surgical suspicion for infection and the uncertain significance of the culture result, treatment options were debated. Medical management was selected based on the presumption that if infection was present, it was a native joint infection in which surgical débridement had already been undertaken at the time of primary arthroplasty. Similar reports for the treatment of M. tuberculosis infection in the knee have been reported with some success.14,15 Short-interval reassessment was planned. Antimicrobial therapy was selected based on susceptibility data and clinical experience and consisted of intravenous (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. Over the ensuing weeks, she developed fevers, knee swelling, and persistent elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). With known potential of this organism for biofilm formation in other areas of the body and positive repeat cultures of the knee joint fluid, confirming the offending organism, a deep and resistant infection of the implant could not be excluded. Therefore, in an attempt to give the patient the best opportunity for clinical cure, the patient subsequently underwent a 2-stage antibiotic spacer explantation and exchange (Figures 3A, 3B). Moderate caseous material was present throughout the knee joint and the subcutaneous tissues. All bone was débrided, and complete synovectomy was undertaken, along with the removal of all implants. The antibiotic concentrations within the spacer were selected by guidance from the Infectious Disease and Pharmacy based on minimal inhibitory concentrations, with 3 packages of cement (40 g each) utilized and a total of 10 g of amikacin and 24 g of cefoxitin contained within the spacer. The patient continued systemic administration of amikacin, cefoxitin, and clarithromycin.
Continue to: One month postoperatively...