PANDAS. Consider a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS) when tics present abruptly with upper respiratory tract illness.10 In this context, throat culture and antibody titers for group A beta hemolytic streptococcal infection may be warranted. Treat aggressively with antibiotics such as penicillin V when tests are positive.
- Assess for comorbid illnesses, then prioritize and treat the most troublesome symptoms
- Aim to decrease rather than eliminate tic-related discomfort
- Medicate only if tics cause distress and dysfunction
- Use one agent when needed at the lowest effective dosage to minimize side effects and drug interactions
- Involve the family and school to monitor progress
- Reassess treatment efficacy often
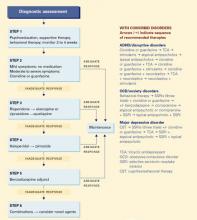
6-STEP TREATMENT APPROACH
A six-step approach—based on our experience and available evidence—can guide treatment. Tics coexisting with ADHD/disruptive disorders, OCD/anxiety disorders, or major depressive disorder call for specialized strategies (Algorithm).
Step 1: Nondrug therapies. Psychoeducation, supportive therapy, and behavioral therapy are appropriate for all patients with burdensome tics. The unusual behaviors associated with tic disorders may have a far-reaching impact on a child’s functioning, self-esteem, and confidence. These effects can be moderated when children and their families understand tics’ fluctuating nature, including their:
- increase with stress and fatigue
- capacity for brief inhibition
- and high rate of spontaneous remission.
Because of this fluctuating pattern, observe the patient for a few weeks before starting medical treatment, unless dysfunction is severe. Observation is especially useful for initial presentations, in which symptom peaks tend to precede quiescence. Carefully weigh the benefits and potential risks of medical treatment for each patient.
Behavioral options include habit reversal, relaxation training, and self-monitoring. One of the few studies of behavioral therapy found tic symptoms decreased 55% with habit reversal, 44% with self-monitoring, and 32% with relaxation training.11
Step 2: Adrenergic alpha-2 agonists. If medication seems appropriate for moderate to severe tics, we recommend clonidine or guanfacine (Table 2) as first-line therapy. These agents decrease the release of norepinephrine, dopamine, and gluta-mate, and norepinephrine turnover.12 They are commonly used to treat Tourette’s disorder because they are better tolerated than antipsychotics, although controlled studies supporting their use are limited and the FDA has not approved this indication.
Approximately one-fourth of Tourette’s disorder patients respond well to clonidine.6 Pulse and blood pressure need to be monitored when using these medications, which in rare cases cause hypotension, bradycardia, and cardiac conduction delay. Because of its sedating properties, clonidine is frequently given at bedtime to promote sleep. Use caution when giving clonidine with medications that have potential cardiovascular effects—such as propranolol or tricyclic antidepressants.
Guanfacine is similar to clonidine except that it binds alpha-2a receptors more selectively and has a longer half-life. As such, it is associated with lower rates of sedation and hypotension than clonidine.
Step 3: Atypical antipsychotics. If symptoms do not respond adequately to an adrenergic alpha-2 agonist, try an atypical antipsychotic. Atypicals block dopamine (D2) receptors and—as a result of serotonergic-2 blockade—are less likely to cause extrapyramidal symptoms than are older antipsychotics.
Risperidone, the most studied atypical in Tourette’s disorder, has been shown to reduce symptoms by 21 to 61%—an effect significantly greater than placebo13 and similar to that of pimozide14 and clonidine. Because it is also relatively well-tolerated, a risperidone trial is warranted before using typical antipsychotics. Tics may worsen during withdrawal while switching a patient from a typical to an atypical antipsychotic.
In one comparative, crossover study in adults with severe Tourette’s disorder, olanzapine was more effective at 5 and 10 mg/d than pimozide at 2 and 4 mg/d, respectively.15 Weight gain and abnormal glucose tolerance associated with olanzapine may be troublesome side effects. Ziprasidone has demonstrated a 35% decrease in tic symptoms in placebo-controlled studies.16 Its use has been associated with increased risk of QTc interval prolongation, requiring ECG monitoring.
Two case series have reported positive effects when quetiapine was used for tics and Tourette’s disorder.17 Like clozapine (which is ineffective for tics), quetiapine has relatively low D2 antagonist potency, suggesting its efficacy in treating tics may be limited. Unlike clozapine, however, quetiapine has few anticholinergic effects. Aripiprazole’s pharmacodynamic profile suggests similar efficacy, but its use in tic disorders has not been validated.
Algorithm 6-step treatment approach to tics and Tourette’s disorder
Further controlled trials of atypical antipsychotics in children and adolescents with tic disorders are needed. ECGs are recommended to monitor QTc intervals when using these medications.
Step 4: Typical antipsychotics. Haloperidol is the most commonly used medication for treating pediatric Tourette’s disorder13 and one of two drugs (pimozide is the other) approved by the FDA for this indication. These postsynaptic D2 antagonists are the most-studied and most-potent medications for treating tics and Tourette’s disorder. Many other typical antipsychotics such as fluphenazine, thioridazine, trifluoperazine, molindone, and thiothixene also have been used.