- lack of efficacy (patient was switched to another drug assigned at random)
- lack of tolerability (patient requested a drug change)
- safety problem (investigator initiated a switch)
- patient’s decision for any reason (often dropping out of the study).
The longer subjects stayed on the first antipsychotic they received, the more effective that drug was considered to be.
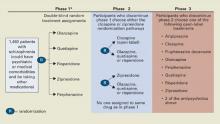
Figure CATIE schizophrenia trial design
* Phase 1A: participants with tardive dyskinesia (N=231) do not get randomized to perphenazine; phase 1B: participants who fail perphenazine will be randomized to an atypical (olanzapine, quetiapine, or risperidone) before eligibility for phase 2.
Source: Reference 2.Medications. Three atypicals—risperidone, olanzapine, and quetiapine—were approved for schizophrenia when the trial began in 1999. Recruitment ended in June 2003, the last subject completed the 18-month trial in December 2004, and data analysis began in January 2005. Ziprasidone was added to phase 1 after 40% of the sample had been enrolled, and aripiprazole was included as an option in the unblinded phase 3.
Perphenazine was chosen to represent typical antipsychotics because it has medium potency and less risk of EPS than high-potency drugs such as haloperidol and is associated with less weight gain than low-potency drugs such as thioridazine.
Dosing. Pharmaceutical manufacturers donated the antipsychotics and were invited to recommend their respective drugs’ starting dosages, dose increments, and maximum dosages. Olanzapine’s maker requested a higher starting dosage (7.5 mg/d instead of 5.0 mg/d) and a maximum dosage 50% higher than the FDA-approved range (30 mg/d instead of 20 mg/d). The others recommended the FDA-approved dosage ranges or less:
- quetiapine, 200 to 800 mg/d
- risperidone, 1.5 to 6 mg/d
- ziprasidone, 40 to 160 mg/d
- perphenazine, 8 to 32 mg/d.
The study team accepted their recommendations.
The medications were packaged in identical capsules. Quetiapine and ziprasidone were given twice daily because of product labeling; risperidone, olanzapine, and perphenazine were given once daily to one-half the patients assigned to them and twice daily to the others to prevent raters from guessing which drug a patient was receiving.
Tardive dyskinesia. For ethical reasons, the 231 patients with TD at enrollment were randomly assigned in phase 1 to atypicals but not to perphenazine because of the well-established link between typical antipsychotics and TD. This exception could have contributed to the closer-than-expected differences in EPS and perhaps in efficacy, given reports that TD patients have more negative symptoms and cognitive dysfunction.13 However, a statistical analysis took that into account.
CATIE’s Key Findings
Discontinuation. A disappointingly high discontinuation rate (74% overall) within a few months was the most important finding (Table 3). A recent effectiveness study with a design similar to the CATIE trial found a similarly high rate of all-cause discontinuation (70%) in patients with first-episode psychosis.14 Thus, patient-initiated drug discontinuation appears to be a core illness behavior from schizophrenia onset to chronic illness.
The high discontinuation rate shows that we need to modify our approach to schizophrenia, emphasizing full adherence to antipsychotic therapy from the onset of the illness.
Table 3
All-cause discontinuation rates in the CATIE trial
| Antipsychotic | Percent discontinued | Duration on antipsychotic (months)* | Dosage (mg/d)* |
| Olanzapine | 64% | 9.2 | 20.1 |
| Perphenazine | 75% | 4.6 | 20.8 |
| Quetiapine | 82% | 4.8 | 543.4 |
| Risperidone | 74% | 5.6 | 3.9 |
| Ziprasidone | 79% | 3.5 | 112.8 |
| Overall | 74% | Median 6.0; mean 8.3 | |
| Notes | |||
| *Mean modal | |||
| Olanzapine’s discontinuation rate was significantly lower than those of perphenazine, quetiapine, and risperidone but not of ziprasidone. | |||
| Olanzapine’s maximum dosage was 30 mg/d (50% higher than FDA-approved 20 mg/d); other agents were dosed within approved ranges. | |||
| Patients reached maximum daily antipsychotic dosages at these rates: 40% with olanzapine, 40% with perphenazine, 44% with quetiapine, 40% with risperidone, and 48% with ziprasidone. | |||
Effectiveness—measured as all-cause discontinuation or switching—was the primary outcome of phase 1. The unexpected finding that perphenazine and the atypicals had similar effectiveness could influence clinical practice. Insurers, for example, might consider promoting cheaper typical antipsychotics for first-line use. CATIE’s cost-effectiveness arm (Rosenheck et al, submitted for publication) will provide additional data on this issue.
Before rushing to use older antipsychotics as first-line treatments for schizophrenia, however, policymakers should consider three factors in the study design that could have enhanced perphenazine’s efficacy and safety profiles.
First, perphenazine was given at lower dosages (up to 32 mg/d) than “real world” clinicians used a decade ago (up to 64 mg/d). Thus, lower rates of serious side effects, especially TD, might have occurred in the study than in past clinical practice. Since atypical antipsychotics were approved, clinicians see far fewer psychiatric patients with pill-rolling tremors, rigid posture, or a shuffling gait, compared with 10 to 15 years ago when typical antipsychotics were widely used.
Second, perphenazine was associated with the highest EPS rate (17%), though its mean modal dosage (20.8 mg/d) is considered moderate. Discontinuation because of EPS was highest with perphenazine and lowest with quetiapine.