Fluoxetine. Seven autistic patients ages 9 to 20 were treated with fluoxetine, 20 to 80 mg/d (mean 37.1 mg/d) in an 18-month longitudinal open trial. Irritability, lethargy, and stereotypy improved, as measured by the ABC.13 Adverse effects included increased hyperactivity and transient appetite suppression.
In another open-label trial of 37 autistic children ages 2 to 7, response was rated by investigators as “excellent” in 11 and “good” in another 11 who received fluoxetine, 0.2 to 1.4 mg/kg/d. However, aggression was a frequent cause for drug discontinuation during the 21-month study.14
Sertraline. In an open-label trial, Steingard et al treated nine autistic children ages 6 to 12 with sertraline, 25 to 50 mg/d for 2 to 8 weeks. Clinical improvement was observed in irritability, transition-associated anxiety, and need for sameness in eight of the children.15
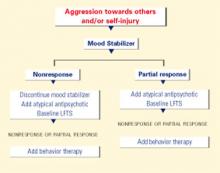
Figure 2 BEHAVIOR-BASED DRUG THERAPY FOR POSTPUBERTAL PATIENTS WITH AUTISM
Source: Reprinted with permission from McDougle CJ, Posey DJ. Autistic and other pervasive developmental disorders. In: Martin A, Scahill L, et al (eds). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2002. Copyright © 2002 by Oxford University Press, Inc.In an open-label trial, 42 adults with pervasive developmental disorders were treated for 12 weeks with sertraline, 50 to 200 mg/d (mean 122 mg/d). Twenty-four (57%) achieved CGI ratings of “much improved” or “very much improved” in aggressive and repetitive behavior.16
Paroxetine. Fifteen adults with profound mental retardation (including seven with pervasive developmental disorders) received paroxetine, 10 to 50 mg/d (mean 35 mg/d) in a 4-month open-label trial. Frequency and severity of aggression were rated as improved after 1 month, but not at 4 months.17
Mirtazapine. In an open-label trial, 26 patients ages 3 to 23 with pervasive developmental disorders were treated with mirtazapine, 7.5 to 45 mg/d (mean 30.3 mg/d). Aggression, self-injury, irritability, hyperactivity, anxiety, depression, and insomnia decreased in 9 patients (34%), as determined by ratings of “much improved” or “very much improved” on the CGI. Adverse effects included increased appetite and transient sedation.18
Mood stabilizers
Lithium. No recent studies of lithium in pervasive developmental disorders are known to exist, but several case reports have been published:
- two reports found lithium decreased manic symptoms in individuals with autism and a family history of bipolar disorder19,20
- one report of lithium augmentation of fluvoxamine in an adult with autistic disorder noted markedly improved aggression and impulsivity after 2 weeks of treatment, as measured by the CGI, Brown Aggression Scale, and Vineland Adaptive Behavior Scale.21
Divalproex. In an open-label trial, 14 subjects ages 5 to 40 with pervasive developmental disorders were given divalproex sodium, 125 to 2,500 mg/d (mean 768 mg/d; mean blood level 75.8 mcg/mL. Affective instability, repetitive behavior, impulsivity, and aggression improved in 10 patients (71%), as measured by CGI ratings of “much improved” or “very much improved.”22 Adverse effects included sedation, weight gain, hair loss, behavioral activation, and elevated liver enzymes.
Lamotrigine. In a 4-week, double-blind, placebo-controlled trial, lamotrigine, 5.0 mg/kg/d, was given to 14 children ages 3 to 11 with autistic disorder.23 Lamotrigine and placebo showed no significant differences in effect, as measured by the ABC, Autism Behavior Checklist, Vineland scales, Childhood Autism Rating Scale, and PreLinguistic Autism Diagnostic Observation Scale. Insomnia and hyperactivity were the most common adverse effects.
α2-adrenergic agonists
Clonidine. Jaselskis et al administered clonidine, 4 to 10 mcg/kg/d, to eight children ages 5 to 13 with autism in a 6-week, double-blind, placebo-controlled, crossover study.24 Symptoms of hyperactivity, irritability, and oppositional behavior improved, as determined by teacher ratings on the ABC, Conners Abbreviated Parent-Teacher Questionnaire, and Attention Deficit Disorder with Hyperactivity Comprehensive Teacher’s Rating Scale. Adverse effects included hypotension, sedation, and decreased activity.
Nine autistic patients ages 5 to 33 received transdermal clonidine, 0.16 to 0.48 mcg/kg/d (mean 3.6 mcg/kg/d), in a 4-week, double-blind, placebo-controlled, crossover study. Hyperarousal, impulsivity, and self-stimulation improved, as measured by the Ritvo-Freeman Real Life Rating Scale and the CGI. Sedation and fatigue were reported, especially during the first 2 weeks of treatment.25
Guanfacine. The effect of guanfacine, 0.25 to 9.0 mg/d (mean 2.6 mg/d), was examined retrospectively in 80 children and adolescents ages 3 to 18 with pervasive developmental disorders.26 Hyperactivity, inattention, and tics improved in the 19 subjects (23.8%) who were rated “much improved” or “very much improved” on the CGI. Sedation was the most frequently reported adverse effect.
Psychostimulants
Early reports showed little benefit from stimulants in pervasive developmental disorders, but more recent studies suggest a modest effect. The RUPP Autism Network is conducting a large controlled investigation of methylphenidate to better understand the role of stimulants in children and adolescents in this diagnostic cluster.