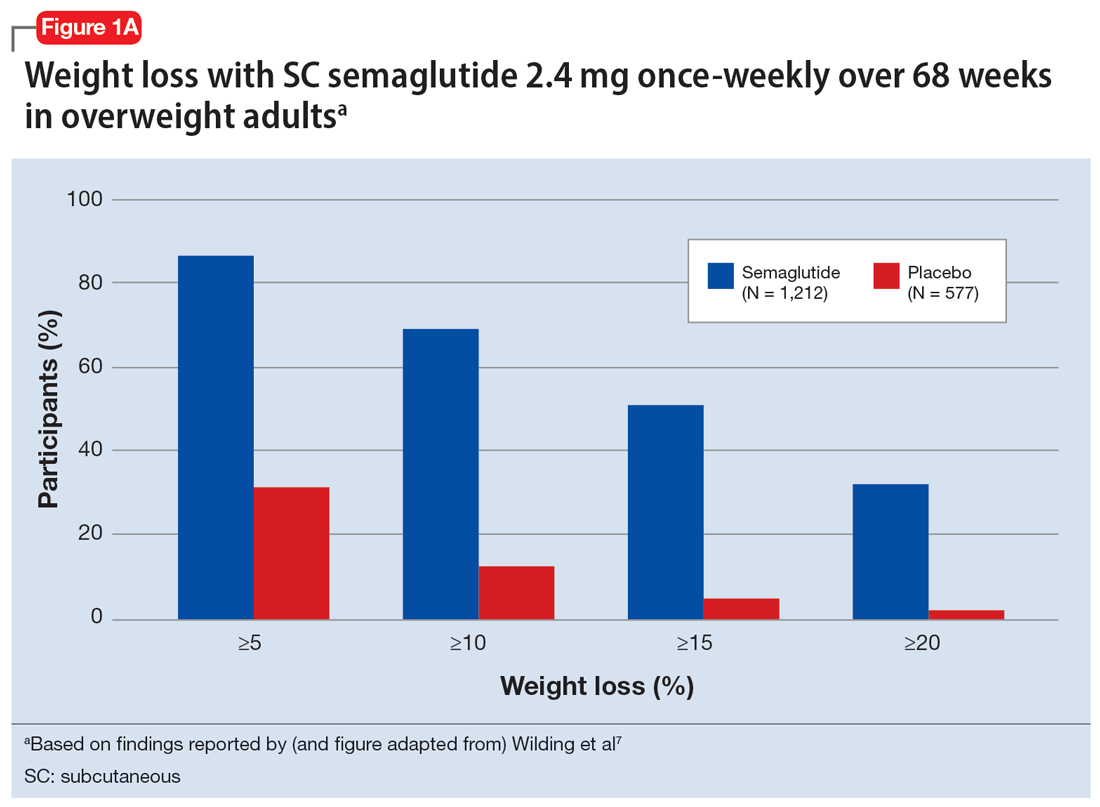
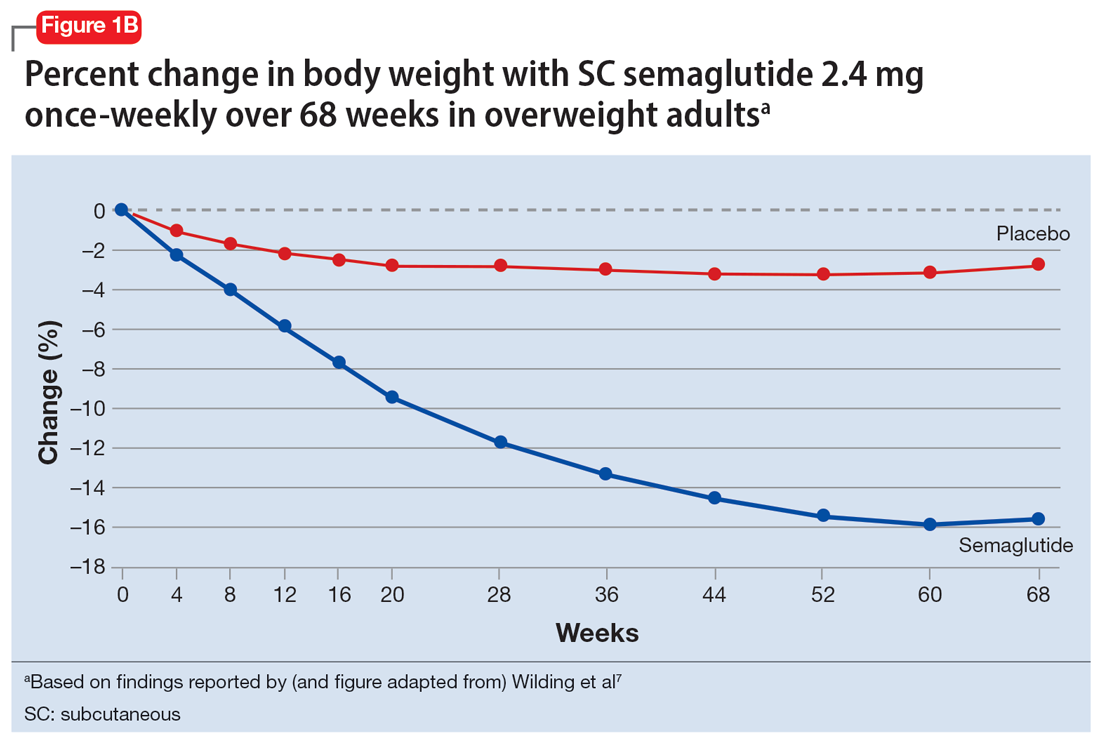
In studies of semaglutide for overweight/obese patients with type 2 diabetes or prediabetes, clinical trials of oral semaglutide (Rybelsus) found a mean weight loss over 26 weeks of -1.0 kg with dosing at 7 mg/d and -2.6 kg with dosing at 14 mg/d.6 A 68-week placebo-controlled trial of semaglutide (dosed at 2.4 mg SC weekly) for overweight/obese adults who did not have diabetes yielded a -15.3 kg weight loss (vs -2.6 kg with placebo); one-half of those who received semaglutide lost 15% of their initial body weight (Figure 1A and Figure 1B).7 Similar findings with semaglutide 2.4 mg SC weekly (Wegovy) were observed in overweight/obese adolescents, with 73% of participants losing ≥5% of their baseline weight.8 A comparative randomized trial in patients with type 2 diabetes also found modestly but significantly greater weight loss with oral semaglutide than with SC liraglutide.9
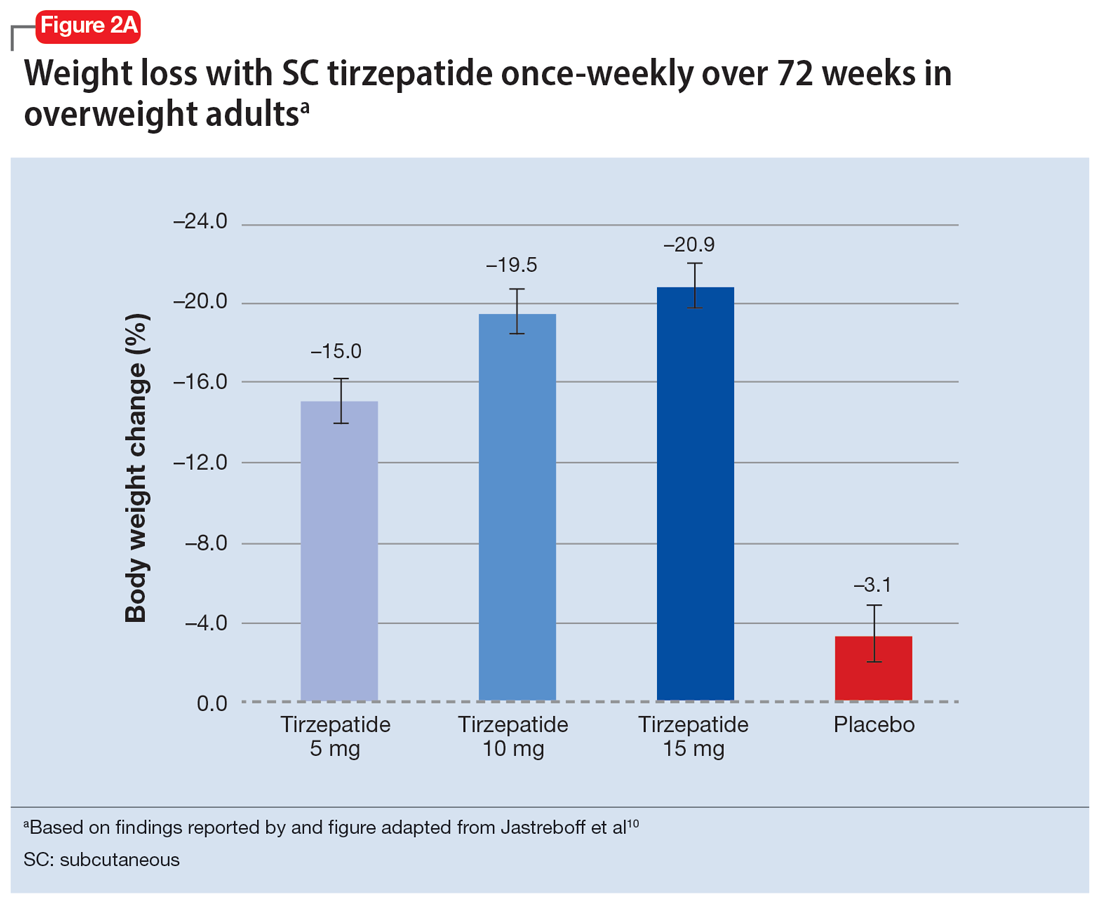
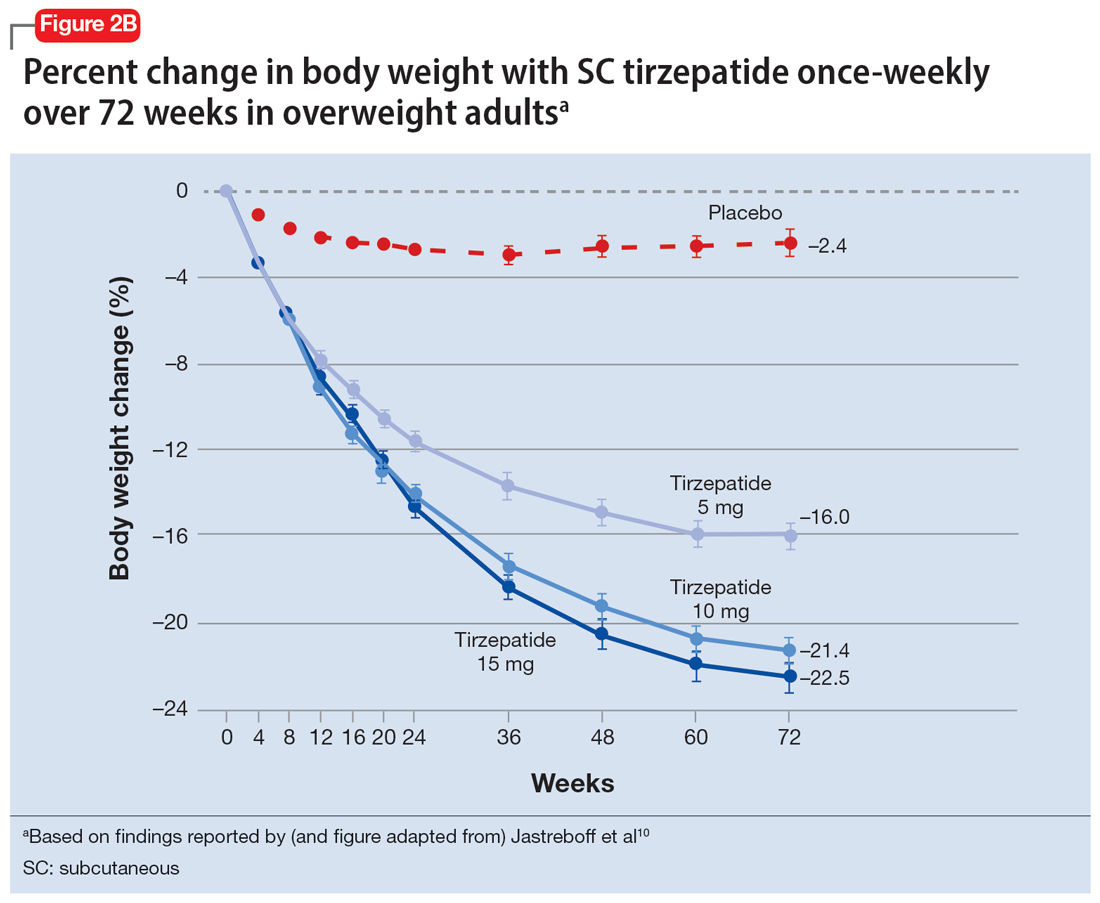
In a 72-week study of tirzepatide specifically for weight loss in nondiabetic patients who were overweight or obese, findings were especially dramatic (Figure 2A and Figure 2B).10 An overall 15% decrease in body weight was observed with 5 mg/week dosing alongside a 19.5% decrease in body weight with 10 mg/week dosing and a 20.9% weight reduction with 15 mg/week dosing.10 As noted in Figure 2B, the observed pattern of weight loss occurred along an exponential decay curve. Notably, a comparative study of tirzepatide vs once-weekly semaglutide (1 mg) in patients with type 2 diabetes11 found significantly greater dose-dependent weight loss with tirzepatide than semaglutide (-1.9 kg at 5 mg, -3.6 kg at 10 mg, and -5.5 kg at 15 mg)—although the somewhat low dosing of semaglutide may have limited its optimal possible weight loss benefit.
Tolerability
Adverse effects with GLP-1 agonists are mainly gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or constipation)5-11 and generally transient. SC administration is performed in fatty tissue of the abdomen, thigh, or upper arm; site rotation is recommended to minimize injection site pain. All GLP-1 agonists carry manufacturers’ warning and precaution statements identifying the rare potential for acute pancreatitis, acute gall bladder disease, acute kidney injury, and hypoglycemia. Animal studies also have suggested an increased, dose-dependent risk for thyroid C-cell tumors with GLP-1 agonists; this has not been observed in human trials, although postmarketing pharmacovigilance reports have identified cases of medullary thyroid carcinoma in patients who took liraglutide. A manufacturer’s boxed warning indicates that a personal or family history of medullary carcinoma of the thyroid poses a contraindication for taking semaglutide, liraglutide, or tirzepatide.
Initial evidence prompts additional questions
GLP-1 agonists represent an emerging class of novel agents that can modulate glycemic dysregulation and overweight/obesity, often with dramatic results whose magnitude rivals the efficacy of bariatric surgery. Once-weekly formulations of semaglutide (Wegovy) and daily liraglutide (Saxenda) are FDA-approved for weight loss in patients who are overweight or obese while other existing formulations are approved solely for patients with type 2 diabetes, although it is likely that broader indications for weight loss (regardless of glycemic status) are forthcoming. Targeted use of GLP-1 agonists to counteract SGA-associated weight gain is supported by a handful of preliminary reports, with additional studies likely to come. Unanswered questions include:
- When should GLP-1 agonists be considered within a treatment algorithm for iatrogenic weight gain relative to other antidote strategies such as metformin or appetite-suppressing anticonvulsants?
- How effective might GLP-1 agonists be for iatrogenic weight gain from non-SGA psychotropic medications, such as serotonergic antidepressants?
- When and how can GLP-1 agonists be safely coprescribed with other nonincretin mimetic weight loss medications?
- When should psychiatrists prescribe GLP-1 agonists, or do so collaboratively with primary care physicians or endocrinologists, particularly in patients with metabolic syndrome?
Followers of the rapidly emerging literature in this area will likely find themselves best positioned to address these and other questions about optimal management of psychotropic-induced weight gain for the patients they treat.
Bottom Line
The use of glucagon-like peptide 1 (GLP-1) agonists, a relatively new class of incretin mimetics, has been associated with profound and often dramatic weight loss and improvement of glycemic parameters in patients with obesity and glycemic dysregulation. Preliminary reports support the potential targeted use of GLP-1 agonists to counteract weight gain associated with second-generation antipsychotics.
Related Resources
- Singh F, Allen A, Ianni A. Managing metabolic syndrome in patients with schizophrenia. Current Psychiatry. 2020;19(12):20-24,26. doi:10.12788/cp.0064
- Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821- 2839. doi:10.1007/s12325-021-01710-0
Drug Brand Names
Amantadine • Gocovri
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Liraglutide • Victoza, Saxenda
Metformin • Glucophage
Naltrexone • ReVia
Olanzapine • Zyprexa
Olanzapine/samidorphan • Lybalvi
Phentermine • Ionamin
Semaglutide • Rybelsus, Ozempic, Wegovy
Tirzepatide • Mounjaro
Topiramate • Topamax
Zonisamide • Zonegran