WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.
“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
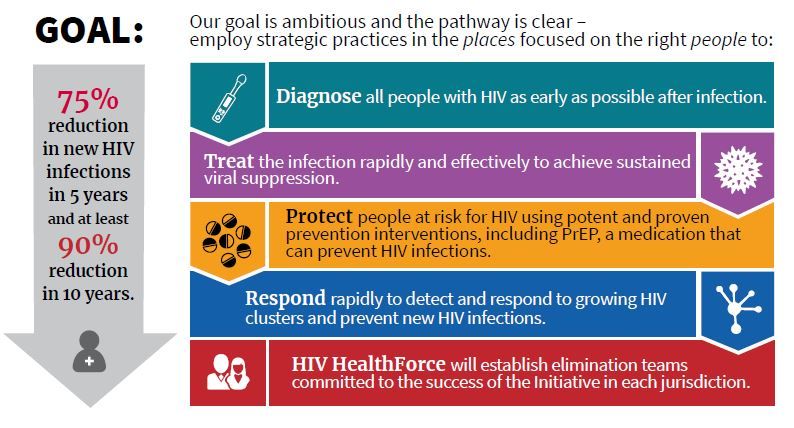
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.