Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079.
HPV screening that distinguishes types 16 and 18 from other oncogenic (high-risk) HPV types identifies women at the greatest risk of CIN 2/3+ and may permit less aggressive management of women with other high-risk HPV infections.
The solution to the dilemma of having to wait 6 to 12 months to repeat Pap and HPV tests for women with a normal cytology but a positive HPV test before determining the need for colposcopy may be solved by type-specific HPV testing. The 10-year cumulative incidence of CIN 3 and cervical cancer (CIN 3+) in 20,810 women tested once for HPV at enrollment was only 0.8% in the women who tested negative for high-risk HPV by Hybrid Capture 2. In contrast, CIN 3+ developed in 17% of the HPV-16-positive women and 14% of the HPV-18-positive women within 10 years.
Women positive for other high-risk types of HPV, but negative for HPV 16 and 18 had far less risk: only 3% developed CIN 3+.
When stratified by age to limit the analysis to women aged 30 and older, the cumulative incidence of CIN 3+ was 20% in HPV-16-positive women and 15% in HPV-18-positive women (FIGURE 1). Contrast these results to the 10-year predictive value of 11% for an LSIL Pap for the same level of cervical neoplasia. In other words, a single positive HPV 16 or 18 test is almost twice as likely to identify women at high risk for CIN 3+ as an LSIL Pap result, over time.
FIGURE 1 Positive HPV 16 or 18 linked to 14% to 17% incidence of CIN3+
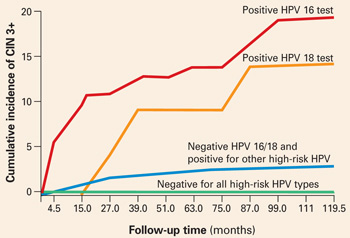
The cumulative incidence of CIN 3+ over a 10-year period, as a function of a single HPV test result at enrollment. Women positive for HPV 16 or 18 had a much greater incidence of CIN 3+, compared to women negative for HPV 16 and 18 but positive for other high-risk HPV types by Hybrid Capture 2, or negative for all high-risk HPV types. Adapted from Khan et al.
Follow-up according to risk
These findings support a follow-up strategy that would permit risk stratification of HPV-infected women for whom an optimal repeat screening interval has been unclear.
- Women positive for HPV 16 or 18 warrant referral to colposcopy, for they carry the majority of risk from a positive high-risk HPV test.
- Women positive only for other high-risk types could be reassured of the safety of a 12-month interval without colposcopy, and referred to colposcopy only if the repeat Pap shows worse than atypical squamous cells of undetermined significance (ASC-US) or the HPV test is again positive (FIGURE 2).
FIGURE 2 Type-specific testing in clinical practice
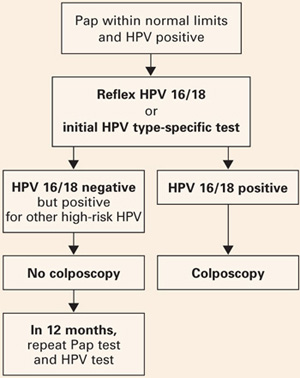
Proposed management of women aged 30 or older, who are screened concurrently with both a Pap test and an HPV test, with typing for HPV 16/18. Adapted from Khan et al.
2 type-specific tests in the pipeline
Currently, the only FDA-approved test for combined screening of women aged 30 and older is the Hybrid Capture 2 High-risk HPV test, which tests for a panel of the 13 most common HPV types known to cause cervical cancer, but does not report on individual types.
But 2 type-specific HPV tests may become available in 2006, which would enable clinicians to follow this strategy.
Digene is nearly ready to launch a 16, 18, 45 type-specific “reflex” test (to a positive Hybrid Capture 2 HPV panel), and Roche is preparing to get its type-specific Linear Array HPV test approved.
2 Better management of screen positives
New practice bulletin on managing abnormal tests
ACOG Practice Bulletin, Number 66. Management of abnormal cervical cytology and histology. Washington, DC: American College of Obstetricians and Gynecologists; September 2005.
The new Practice Bulletin published last September in most respects mirrors the most recent American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus guidelines.1
Key points
- ASC-US may be managed by referral to immediate colposcopy, by repeat Pap, or by HPV testing. However, “reflex HPV testing” when ASC-US is derived from liquid-based cytology has advantages. (It is estimated that a large majority of ASC-US is now managed by HPV testing.)
- Initial management of all other Pap abnormalities is by immediate referral to colposcopy, ie, the finding of atypical squamous cells cannot rule out high-grade (ASC-H), atypical glandular cells (AGC), LSIL, and high-grade intraepithelial lesions (HSIL).
- Management of ASC-US and LSIL in adolescence and postmenopause: ACOG provides an alternative strategy for adolescents with either ASC-US or LSIL cytology, who may have either repeat cytology at 6 and 12 months or a single HPV test at 12 months. ACOG did not differentiate postmenopausal women with either ASC-US or LSIL as “special situations” with additional management strategies.
- CIN 2/3 should usually be treated, both guidelines say. The only exception is the adolescent with CIN 2, who may be followed with repeat cytology and colposcopy at 4 to 6 months if she is deemed reliable for follow-up, the colposcopy is adequate, and the endocervical sampling is negative.
- HPV-positive ASC-US, ASC-H, or LSIL and either CIN 1 or normal colposcopy findings should be followed by repeat Pap at 6 and 12 months, or a single HPV test at 12 months, with referral to colposcopy if either the Pap results show ASC-US or more advanced abnormality or the HPV test is positive.
- In contrast, an excisional procedure is required for normal findings, or an unsatisfactory colposcopy in nonpregnant women referred for atypical glandular cells “favor neoplasia” (AGC-H), or adenocarcinoma in situ (AIS), or repeat atypical glandular cells “not otherwise specified” (AGC-NOS), or HSIL. The only exception is an adolescent with HSIL cytology and a satisfactory and normal colposcopy and biopsy, who may be followed closely.
- Women treated for CIN 2/3 can be monitored after treatment by cytology screening at 6-month intervals 3 or 4 times or by a single HPV test at 6 months, before returning to annual screening. Any repeat abnormal Pap at the threshold of ASC-US or more advanced abnormality or a positive HPV test requires colposcopic evaluation.


