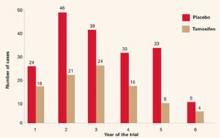
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases per 1,000, compared with 6.8 cases per 1,000 in the placebo group. Overall, the reduction in invasive breast cancer was 49% (P<.000001). The reductions were 44% for women in the group aged 35 to 49 years, 51% for those aged 50 to 59, and 55% for those 60 years and older (FIGURE 2).
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ) by 50%. (Expanded use of mammography has led to greater detection of this cancer. Most such lesions are estrogen-receptor–positive.8) In addition, tamoxifen reduced breast cancer risk in women with a history of lobular carcinoma in situ by 56% and atypical hyperplasia by 86%. Overall, tamoxifen decreased the occurrence of estrogen-receptor–positive tumors by 69%, but had no impact on tumors that were estrogen-receptor–negative.
Tamoxifen’s other effects in healthy women. The BCPT offered the first largescale data on the effects of tamoxifen in healthy women.7 (All previous studies included only women with breast cancer.) Several secondary endpoints merit consideration.
- Endometrial cancer risk. Researchers found the relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence intervals [CI], 1.35, 4.97). When this figure was calculated for the different age groups, it rose to 4.01 (95% CI, 1.70, 10.90) in women over 50, and declined to 1.21 for women ages 49 and under (95% CI, 0.41, 3.60).
- Thromboembolic event risk. The same age distinction was seen in relation to thromboembolic events. There were no statistically significant increases in pulmonary emboli or deep venous thrombosis in women 49 years of age or under. Although it is unclear whether the trial was sufficiently powered for this particular endpoint, the likelihood that serious adverse events will limit the potential benefits of tamoxifen appears to be lower in women under the age of 50. This has significant clinical consequences for physicians caring for perimenopausal patients.
- No change in incidence of other cancers. Overall, the incidence of invasive cancers other than those of the breast and uterus was the same for the tamoxifen and placebo groups.
- Other outcomes. The relative risk of death from any cause was 0.81 (95% CI, 0.56–1.16).
There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the development of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these was statistically significant.
The overall relative risk of fractures at various sites (hip, spine, radius) was 0.81 (95% CI, 0.63–1.05).
A statistically significant increase was found in the number of women with cataracts who then underwent cataract surgery. That relative risk was 1.57 (95% CI, 1.16–2.14).
FIGURE 2 Breast Cancer Prevention Trial: Tamoxifen reduces incidence of breast cancer
The rate of cancer reduction for tamoxifen compared with placebo for years 1 through 6 was 33%, 55%, 39%, 49%, 69%, and 55%, respectively.
Reprinted from Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst.1998;90(18):1371-1388, by permission of Oxford University Press.
FDA approves tamoxifen for primary prevention
Based on the BCPT results, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for primary prevention of breast cancer in women at high risk for the disease. It recommended that tamoxifen be limited to high-risk women because of the potentially serious side effects seen in clinical trials.
The FDA did not define high risk, but recommended that prophylactic use of tamoxifen be based on a thorough evaluation of a woman’s personal, family, and medical histories, as well as her age and understanding of the risks and benefits of treatment.
In addition, the FDA required that the package insert advise women to consult a health-care professional for breast cancer risk assessment and state that only women at high risk should take the drug (again, without defining high risk).
In 2002, the FDA added a “black box” warning to tamoxifen labeling that was directed at use of the drug for prevention rather than treatment. This warning concerned the occurrence of uterine sarcomas. The incidence of these cancers was found to be 0.17 per 1,000 women taking tamoxifen, compared with 0.015 per 1,000 controls.9
Underuse of tamoxifen? A recent study by Freedman et al1 calculated the number of women 35 to 79 years of age who were eligible for tamoxifen chemoprevention based on FDA criteria. They further calculated the number of women who would have a positive benefit-risk ratio for tamoxifen use, and concluded that, among white women alone, roughly 28,492 additional breast cancers could be prevented or deferred if these individuals took tamoxifen for the next 5 years.