Preparing the catheter. Using sterile technique, cut the tip from the 16-Foley catheter just beyond the balloon to allow free egress for the stylet. Perform a leak test by filling the balloon with the standard 30 mL of physiologic saline. Withdraw the saline from the balloon and insert the urologic sound into the catheter.
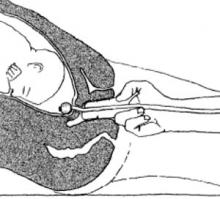
Foley placement. Hold the trimmed Foley catheter with internal stylet between the first 2 fingers of your dominant hand. Then insert the fingers into the patient’s vagina up to the cervix. Position a finger at either side of the cervical opening and place the stylet into the os until it touches the fetal vertex (FIGURE 2). If the catheter does not slide in with the stylet, advance the catheter over the stylet into the extra-amniotic space, using the stylet as a rigid splint to assist in the insertion. Maneuver the tip slightly laterally and inflate the balloon partially while simultaneously withdrawing the stylet. By the time the full 30 mL of physiologic saline has been instilled, the stylet should be completely removed.
Testing insertion. Use your fingers to check that the Foley balloon is completely within the extra-amniotic space. A gentle tug will ensure placement above the internal os. Then push the catheter plug into the open end of the Foley and secure the catheter loosely to the patient’s thigh for comfort. Generally, it is unnecessary to place traction on the Foley catheter to effect effacement and dilatation. If the Foley is not spontaneously expelled, deflate the balloon and remove the catheter within 5 to 12 hours, depending on the time of induction.
FIGURE 2 Tactile technique of Foley balloon insertion
Advantages
Cost. For disposable, off-the-shelf catheters, current hospital costs are less than $10. The Van Buren Curve Catheter Stylet (C.R. Bard Inc, Bard Urologic Division, Covington, Ga) is $22 and can be reused after sterilization.
Mechanical agents. The simplified catheter technique is certainly easier than the insertion of multiple laminaria. Laminaria tents are said to require no monitoring post insertion since they produce no uterine contractions and initiate gradual cervical dilatation by mechanical swelling and displacement; thus, they may be used for outpatient ripening and are safe in vaginal birth after cesarean attempts. However, they have been associated with increased postpartum and neonatal infections, along with traumatic insertions and vaginal bleeding. In addition, oxytocin is almost always necessary to initiate labor.35 The Foley catheter, on the other hand, does cause uterine contractions, but has been associated with a lower rate of tachysystole (12.7%) when compared with misoprostol (38.4%).16
While the Foley balloon requires intermittent monitoring—and continuous monitoring should labor become active27—it is still safe to use after ruptured membranes or in a trial of labor following a previous cesarean.36 No common side effects (intrapartum or postpartum fever and vaginal bleeding,12,18,19,24,26 the quite-rare rupture of membranes, along with displacement of the presenting part and umbilical cord prolapse6,21,37) have been seen with this simplified insertion technique.
Pharmacologic agents. Intracervical or intravaginal prostaglandin E2 (dinoprostone) and oral or intravaginal prostaglandin E1 (misoprostol) are effective and simple to administer. However, these agents are not readily reversible; require continuous monitoring once administered; and are fraught with adverse effects, including fever, nausea and vomiting, diarrhea, and hyperstimulation that may lead to tachysystole, uterine rupture, and fetal morbidity and mortality.39-42
Conclusion
Until further controlled studies documenting outpatient safety are available, balloon ripening should be limited to an in-hospital technique, as it causes uterine contractions and even tachysystole.18,4 In the meantime, clinicians should feel confident including this simplified technique in their obstetric armamentarium.
Dr. Freedman reports no affiliation or financial arrangement with any of the companies that manufacture drugs or devices in any of the product classes mentioned in this article.