Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
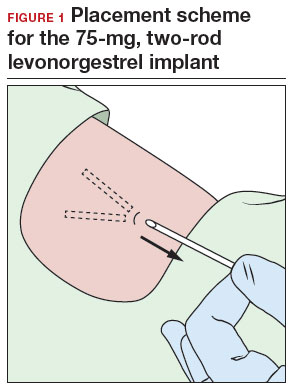
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
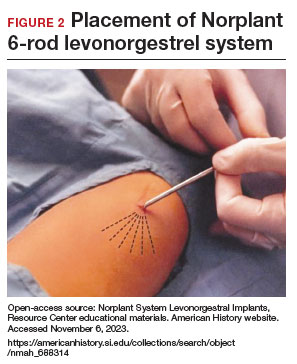
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).
Continue to: How do you approach removal of a deep contraceptive implant?...