New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
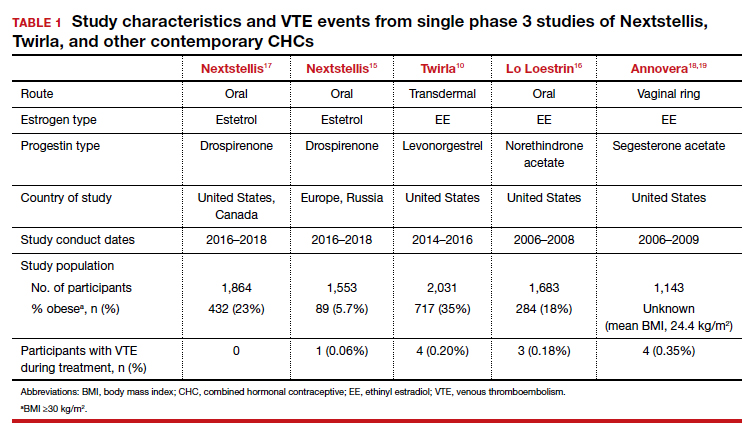
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.
Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...