CASE 1 continued Patient is discharged home
The patient received enoxaparin while she was in the hospital. She is now discharged and doing well. She asks, will she need anticoagulation prophylaxis after delivery?
How would you counsel her?
Chemoprophylaxis in the postpartum period
With no risk of fetal harm and a higher risk of VTE per day, the threshold for chemoprophylaxis is lower in the postpartum period. The risk of postpartum bleeding is less than 1%, with the most common complication being wound hematomas (0.61%).9 For this case patient, the COVID-19 diagnosis does not alter the recommendations for postpartum chemoprophylaxis. Additionally, as the need for neuraxial anesthesia has passed, the use of intermediate-dose chemoprophylaxis over prophylactic-dose is advocated in the postpartum period, especially in obese patients.12
As mentioned previously, there is no standard definition of intermediate-dose. Data suggest that a weight-based intermediate-dose is most likely to achieve therapeutic levels of anti-Xa in this high-risk population compared with a fixed dose.13,14 For example, enoxaparin 0.5 mg/kg twice daily is recommended for patients with class 3 obesity or higher by the Society for Maternal-Fetal Medicine.12
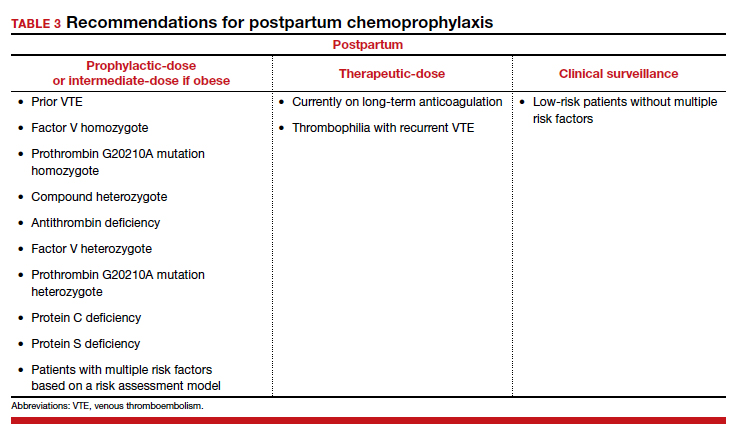
As a rule, anyone who was on chemoprophylaxis antepartum should be continued on at least an equivalent dose for 6 weeks postpartum. Postpartum, patients with any prior DVT should take prophylactic-dose or intermediate-dose chemoprophylaxis for 6 weeks. Patients with a known high-risk thrombophilia should receive prophylactic-dose or intermediate-dose chemoprophylaxis postpartum for 6 weeks. For patients with a low-risk thrombophilia, prophylactic-dose or intermediate-dose chemoprophylaxis is recommended for 6 weeks.
For low-risk patients without prior VTE or thrombophilia, standardized risk assessment is recommended.
Cesarean delivery
Cesarean delivery (CD) is a risk factor for postpartum VTE.9 A universal chemoprophylaxis strategy has not been proven in this patient population. Mechanical prophylaxis with sequential compression devices is recommended for all patients undergoing CD pre-procedure and until patients are fully ambulatory.8,9 Early ambulation also should be encouraged.
Many risk assessment models are available for postoperative VTE prevention, and they have widely different chemoprophylaxis rates. Studies have shown chemoprophylaxis rates of 85% by RCOG, 1% by ACOG, 35% by CHEST, 94% by Caprini, and less than 1% by Padua.15,16 In addition to the antepartum patient-specific risk factors mentioned, postpartum risk factors include infection, postpartum hemorrhage, and transfusion. Based on data extrapolated from the nonobstetric literature, chemoprophylaxis is recommended until discharge from the hospital unless risk factors are expected to continue.9
Neuraxial anesthesia
For patients who require postpartum chemoprophylaxis, the Society for Obstetric Anesthesia and Perinatology (SOAP) offers evidence-based guidelines for use after neuraxial anesthesia. UFH can be initiated 1 hour or longer after a neuraxial procedure and 1 hour or longer after catheter removal. Prophylactic-dose LMWH can be restarted at 12 hours or longer after a neuraxial procedure and at 4 to 6 hours or longer after catheter removal. For patients restarting intermediate-dose or therapeutic-dose, the recommendations are to wait 24 hours or longer after a neuraxial procedure and 4 hours or longer after catheter removal.17 Timing can be individualized based on the patient’s risk of hemorrhage and surgical bleeding. Although it may be tempting to delay chemoprophylaxis in the setting of bleeding, postpartum hemorrhage and transfusion increase the risks of VTE. In this setting, it is best to consider the use of UFH, which safely can be started earlier than LMWH.
For patients without neuraxial anesthesia, ACOG recommends chemoprophylaxis 4 to 6 hours after vaginal delivery and 6 to 12 hours after CD.8 (TABLE 3 summarizes recommendations for postpartum chemoprophylaxis.)
Continue to: Adjusting the anticoagulation regimen...