New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
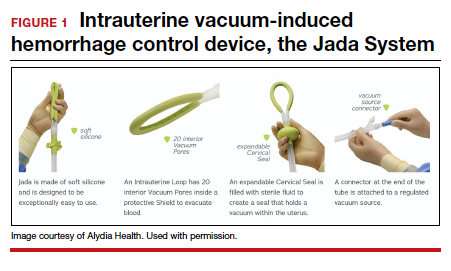
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).
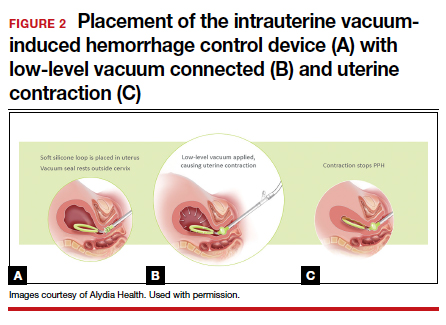
Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.
CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...