Anti-Müllerian hormone
A transforming growth factor β superfamily peptide produced by preantral and early antral follicles of the ovary, anti-Müllerian hormone (AMH) is a direct and quantitative marker of ovarian reserve.18 AMH is detectable at birth; the level rises slowly until puberty, reaching a peak at approximately 16 years of age,19 then remains relatively stable until 25 years, after which AMH and age are inversely correlated, reflecting ongoing oocyte atresia. AMH declines roughly 5% a year with increasing age.14
A low level of AMH (<1 ng/mL) suggests diminished ovarian reserve20,21 (TABLE 1). AMH has been consistently validated only for predicting ovarian response during IVF.2,20 To a lesser extent, AMH might reflect the likelihood of pregnancy following ART, although studies are inconsistent on this point.22 AMH is not predictive of natural fecundity or time to spontaneous conception.3,23 Among 700 women younger than age 40, AMH levels were not significantly different among those with or without infertility, and a similar percentage of women in both groups had what was characterized as a “very low” AMH level (<0.7 ng/mL).14
At the other extreme, a high AMH value (>3.5 ng/mL) predicts a hyper-response to ovarian stimulation with gonadotropins and elevated risk of ovarian hyperstimulation syndrome. In conjunction with clinical and other laboratory findings, an elevated level of AMH also can suggest polycystic ovary syndrome. No AMH cutoff for a diagnosis of polycystic ovary syndrome exists, although a level of greater than 5 to 7.8 ng/mL has been proposed as a point of delineation.24,25
Unlike FSH and AFC, AMH is generally considered to be a valid marker of ovarian reserve throughout the menstrual cycle. AMH levels are higher in the follicular phase of the cycle and lower in the midluteal phase, but the differences are minor and seldom alter the patient’s overall prognosis.26-29 As with FSH and AFC, levels of AMH are significantly lower in patients who are pregnant or taking hormone-based medications: Hormonal contraception lowers AMH level by 30% to 50%.17,30,31 Ideally, patients should stop all hormone-based medications for 2 or 3 months (≥2 or 3 spontaneous cycles) before testing ovarian reserve.
#2 Who should have ovarian reserve testing?
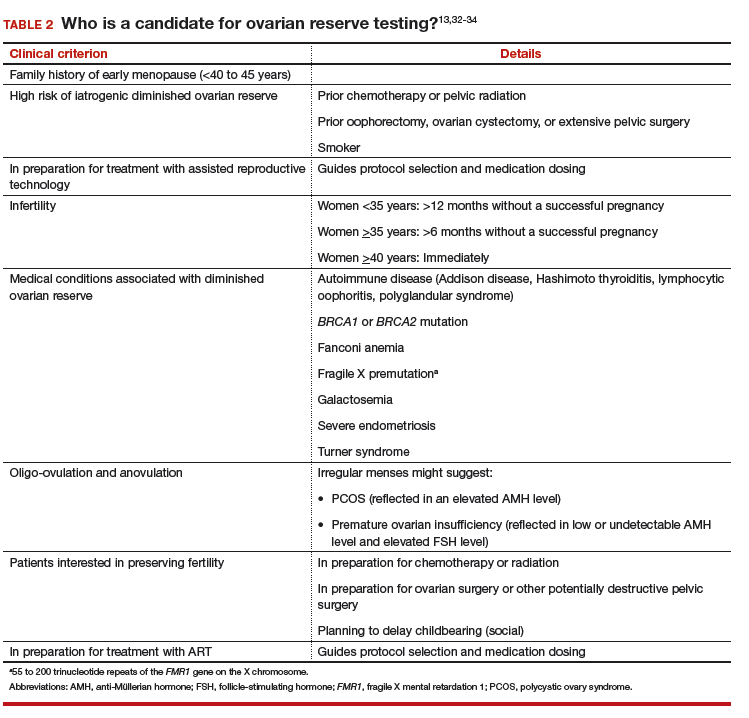
The clinical criteria and specific indications for proceeding with ovarian reserve testing are summarized in TABLE 2.13,32-34 Such testing is not indicated in women who are planning to attempt pregnancy but who do not have risk factors for diminished ovarian reserve. These tests cannot predict their success at becoming pregnant; age is a far more appropriate predictor of pregnancy and risk of miscarriage.3 At most, an abnormal result in a patient who meets one of the clinical criteria for testing could prompt earlier referral to a reproductive specialist for consultation—after it is explained to her that abnormal ovarian reserve tests do not, alone, mean that ART is required.
Continue to: #3 Can I reassure my patient about her reproductive potential using these tests?