Serum biomarkers useful for determining risk, need for referral
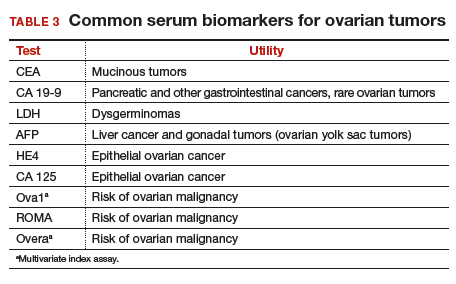
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).
CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
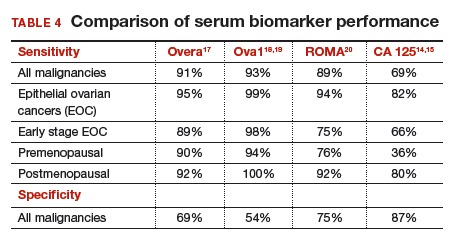
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.
How to evaluate an ovarian tumor
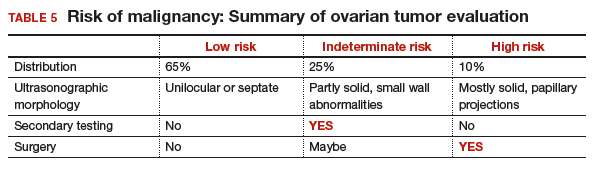
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21
About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
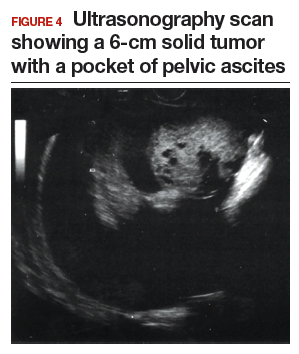
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.
Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
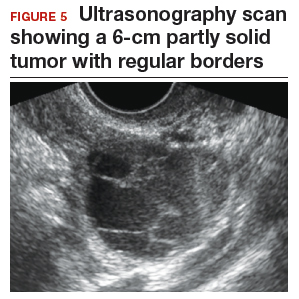
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.
Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.