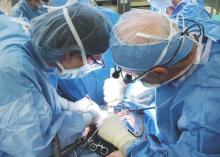
The failure of the first U.S. uterus transplant may have been due a complication triggered by an infection of Candida, according to the Cleveland Clinic.
“Preliminary results suggest that the complication was due to an infection caused by an organism that is commonly found in a woman’s reproductive system,” officials at the Cleveland Clinic said in an April 8 statement. “The infection appears to have compromised the blood supply to the uterus, causing the need for its removal. There is an ongoing review of all the data and the team is modifying the protocol to reduce the chances of this complication occurring again in the future. The health of our patient is and has always been our primary concern.”
A team of surgeons at the Cleveland Clinic performed the first U.S. uterus transplant on a 26-year-old woman with uterine factor infertility on Feb. 24, but had to remove the transplanted uterus several days later following a sudden complication. The transplant is part of a study aimed at achieving pregnancy in women with uterine factor infertility. The study is still ongoing.
mschneider@frontlinemedcom.com
On Twitter @maryellenny