Cerebral palsy (CP) embodies a collection of disorders involving permanent but nonprogressive motor dysfunction secondary to one of a variety of abnormal disturbances that can occur in the developing fetal or infantile brain.1 The motor impairment of CP classically leads to irregularities in muscle tone, posture, and/or movement, resulting in limitations of functional abilities that vary in severity.1,2 Patients with CP commonly experience dysphagia, gastroesophageal reflux disease, impaired airway clearance, chest wall and spine deformities, restrictive lung disease, and/or recurrent aspiration.1 Consequently, pulmonary disease is the leading cause of morbidity and mortality in patients with severe CP, characterized by recurrent bacterial infections.3,4
Frequent antibiotic use increases the risk of multidrug-resistant pathogen formation and hypersensitivity to antibiotics. Life-threatening allergic reactions in a patient population with impaired lung function significantly complicates patient management, often leading to suboptimal treatment with second-line agents.5 This case study describes a previously penicillin-tolerant patient with CP who developed a type I hypersensitivity reaction to ceftazidime/avibactam and was treated successfully with the antibiotic after rapid induction of temporary tolerance.
Case Presentation
A 34-year-old male with a complex medical history of severe spastic CP and atonic seizures was recently diagnosed with adenocarcinoma of the colon and admitted for ileostomy and sigmoidectomy. The surgery was complicated by spillage of intestinal contents into the peritoneal cavity 3 days postoperation. The patient was urgently taken to the operating room for exploratory laparotomy, culminating in remaining colectomy, complete abdominal washout, and wound vacuum placement. He continued to deteriorate clinically over the next few weeks, beginning with the development of feculent peritonitis and septic shock. Respiratory distress ensued, and the patient required a tracheostomy with mechanical ventilation. A computed tomography of the chest was consistent with multifocal pneumonia, and a respiratory culture of bronchioalveolar lavage fluid cultivated Klebsiella pneumoniae, a carbapenem-resistant Enterobacteriaceae.
The infectious disease service was consulted and recommended ceftazidime/avibactam as the only acceptable antibiotic to treat this organism. The patient had no history of drug hypersensitivities. However, he developed diffuse, generalized urticaria and predominately right-sided flushing immediately following the onset of the antibiotic infusion. The urticaria was pruritic. The patient did not have angioedema, and he did not experience any adverse respiratory, cardiac, gastrointestinal, or neurologic symptoms. The infusion was ceased immediately, and the patient was treated with a combination of diphenhydramine 50 mg IV and ranitidine 50 mg IV. Resolution of his hypersensitivity symptoms occurred within an hour of treatment, and vital signs remained stable with no resurgence of symptoms. At the time of his reaction, the patient also was taking pantoprazole, valproate, metoprolol, risperidone, and oxycodone as needed for pain. A tryptase level was not measured.
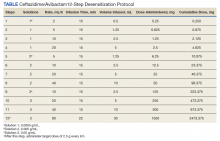
The allergy and immunology service was consulted for rapid desensitization to ceftazidime/avibactam as the culture and sensitivity test demonstrated the bacterium to be resistant to alternative antibiotics. Skin testing to ceftazidime/avibactam was deferred at the time due to the patient’s critical illness. The patient was premedicated with diphenhydramine and ranitidine 50 mg IV. Rapid IV desensitization was performed using a standard 12-step protocol developed for chemotherapeutic agents but demonstrated as safe and effective when applied to antibiotics in patients with cystic fibrosis.5 The antibiotic was administered in sequential 15-minute intervals for a total of 12 progressively doubled doses with continuous monitoring for the appearance of allergic reactions (Table). The target dose of 2.5 g was successfully achieved, and the patient tolerated a complete 14-day treatment regimen with no further adverse reactions to the medication. During the remainder of his hospital admission, the patient improved significantly without further complications.