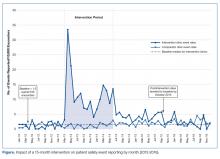
Results
The SER rate was assessed monthly, so the number of SER rates for each group (intervention and comparator) was 15 during the pre-intervention and intervention periods, respectively, and 30 during the postintervention period. During the pre-intervention period, the intervention collaborative’s baseline median rate of safety events reported was 1.5 per 10,000 patient encounters (Figure). Also, for the intervention collaborative, the pre-intervention baseline mean (standard deviation, SD) SER rate (per 10,000 patient encounters by month) was 1.3 (1.2), the intervention mean SER rate was 12.0 (7.3), and the postintervention rate was 3.2 (1.8). Based on the ITS analysis, there was a significant change in the SER rate between the intervention and postintervention periods for the intervention collaborative (P = 0.01).
The SER rate peaked in the first month following the start of the intervention. After discontinuation of feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention (Figure). The postintervention SER rate was also significantly higher than the pre-intervention rate (P = 0.001).
For the comparator clinics, no significant change in SER rates occurred for the 3 time periods.
Discussion
In this initiative with a 5-year reporting window, we had previously shown that with education and prospective audit and feedback, we could achieve a 10-fold increase in patient SER rates among a multi-practice collaborative while the intervention was maintained [1]. Even though there was a modest but significant increase in the SER rate in the postintervention period for the 6-clinic intervention collaborative compared to baseline, the substantial gains seen during the course of the intervention were not maintained when monthly audit and feedback ceased and monitoring continued for 30 months.
Limitations of this study include possible selection bias resulting from including clinics felt likely to participate rather than identifying clinics in a random fashion. In addition, we did not attempt to determine the specific reasons for the decrease in reporting among these clinics.