In this paper, we assess the attainment of diabetes quality measures among patients in this study, specifically, measures of glycemic and BP control.
Methods
Study Design and Patients
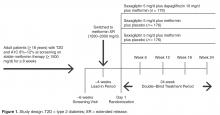
This was a post hoc analysis of a phase 3, multicenter, randomized, double-blind, active-controlled, parallel-group, 24-week study. Details of the study design, inclusion/exclusion criteria, and primary results have been previously reported [15]. In brief, men and women aged ≥ 18 years with T2D poorly controlled (A1C 8.0%–12.0%) with metformin monotherapy were randomized 1:1:1 to receive saxagliptin 5 mg/d and dapagliflozin 10 mg/d, saxagliptin 5 mg/d and placebo, or dapagliflozin 10 mg/d and placebo on a background of metformin extended release 1500 to 2000 mg/d (Figure 1) . Patients were required to be on stable metformin (≥ 1500 mg/d) for ≥ 8 weeks before screening and to have a C-peptide concentration ≥ 1.0 ng/mL and a body mass index ≤ 45.0 kg/m 2. The trial was designed and monitored in accordance with the ethical principles of Good Clinical Practice as defined by the International Conference on Harmonisation and the Declaration of Helsinki. Institutional review boards or ethics committees at each study site approved the protocol, and all patients gave written informed consent.Quality Measure Assessment
Individual measures assessed included the proportion of patients with A1C < 7%, A1C < 8%, A1C > 9%, and BP < 140/90 mm Hg. Composite measures assessed includedthe proportion of patients with A1C < 7% and BP < 140/90 mm Hg and the proportion of patients with A1C < 8% and BP < 140/90 mm Hg.
Antihypertensive or cholesterol-lowering medication use was not controlled for in this study. Patients were maintained on their prescribed dosing regimen for antihypertensive and cholesterol-lowering medications, with adjustments as needed per the standard of care for their diagnosis. Treatment outcomes for A1C < 7%, < 8%, or > 9% were prespecified. The BP treatment outcome was also prespecified per the statistical analysis plan; however, a change to the HEDIS quality measure treatment outcome for BP during the clinical study resulted in this analysis being no longer relevant. Therefore, analyses of the currently endorsed quality measures for BP were conducted post hoc. Quality measure assessments for A1C and BP treatment outcomes were conducted using data from the 24-week, double-blind treatment period.
Statistical Analysis
P values for the differences in proportion of patients with individual treatment outcomes and composite treatment outcomes with saxagliptin plus dapagliflozin plus metformin versus saxagliptin plus metformin or dapagliflozin plus metformin were calculated using Fisher’s exact test. The numerator and denominator for each percentage are the number of responders and the number of patients with non-missing values in the treatment group at the corresponding baseline category, respectively, and are not corrected for baseline A1C. Because some patients experienced improvement in A1C during the lead-in period and could have already been at treatment goal at baseline, a sensitivity analysis excluding these patients was completed. Results are presented for the total number of patients with non-missing values in the treatment group, as well as patients with non-missing values in the treatment group who did not meet quality measure criteria at baseline. The number needed to treat (NNT) was calculated for all comparisons reaching statistical significance.