New vaccine contraindications. Four medical conditions have been added to the list of contraindications for quadrivalent live, attenuated influenza vaccine (LAIV4): cochlear implant, cerebrospinal fluid leak, asplenia (anatomic and functional), and sickle cell anemia.4 In addition, those who receive LAIV4 should not be prescribed an influenza antiviral until 2 weeks after receiving the vaccine. And the vaccine should not be administered for 48 hours after receipt of oseltamivir or zanamivir, 5 days after peramivir, and 17 days after baloxavir marboxil.4 This is to prevent possible antiviral inactivation of the live attenuated influenza viruses in the vaccine.
For those who have a history of severe allergic reaction to eggs, there are now 2 egg-free options: cell-culture-based inactivated vaccine (ccIIV4) and recombinant influenza vaccine (RIV4).3,4 Urticaria alone is not considered a severe reaction. If neither of these egg-free options is available, a vaccine may still be administered in a medical setting supervised by a provider who is able to manage a severe allergic reaction (which rarely occurs).
All vaccine products available for the upcoming influenza season are listed and described on the CDC Web site, as is a summary of related recommendations.4 Particular attention should be paid to the dose of vaccine administered, as it differs by product for those ages 6 through 35 months of age and those ages 65 years and older.
Use of antiviral medications
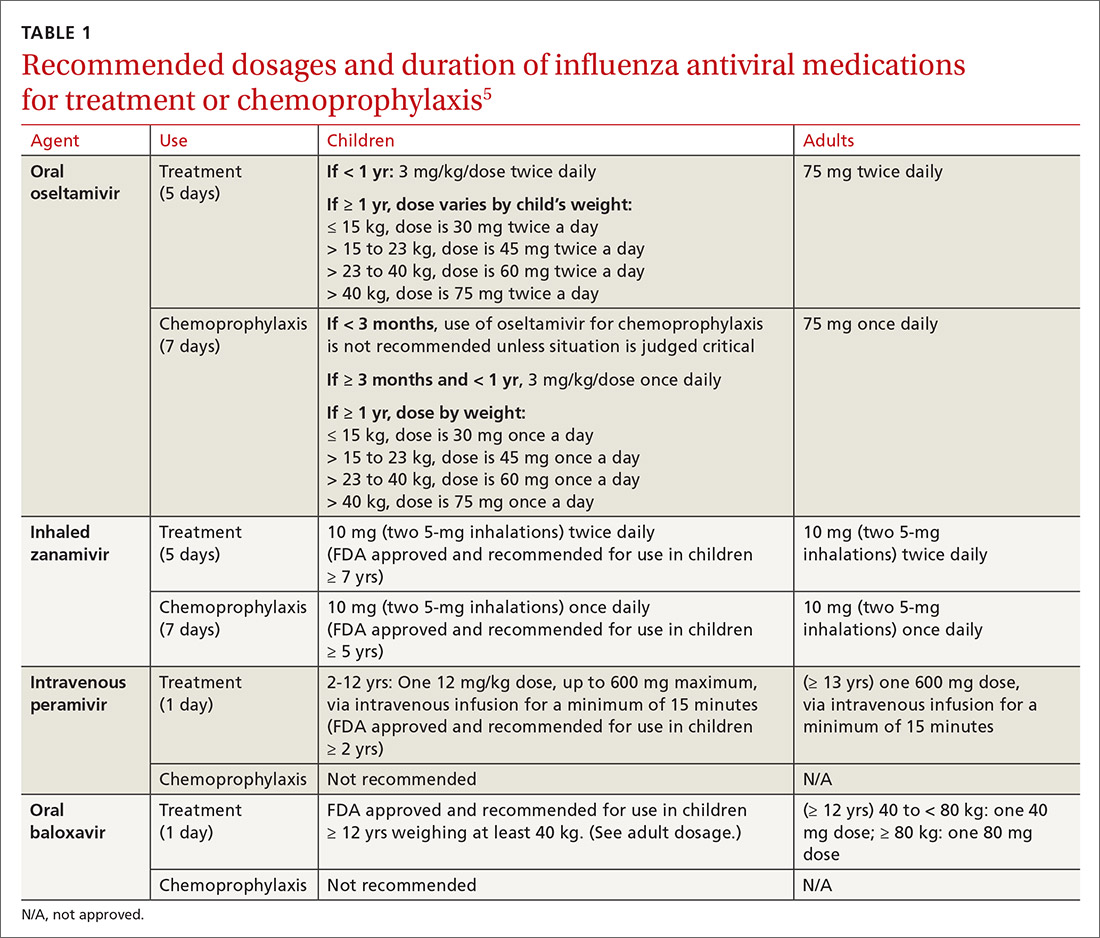
Four antiviral medications are now available for treating influenza (3 neuraminidase inhibitors and 1 endonuclease inhibitor), and there are 2 agents for preventing influenza, both neuraminidase inhibitors (TABLE 1).5 The CDC recommends treating with antivirals as soon as possible if individuals with confirmed or suspected influenza require hospitalization; have severe, complicated, or progressive illness; or are at high risk for complications. Use antivirals based on clinical judgment if previously healthy individuals do not have severe complications and are not at increased risk for complications, and only if the medication can be started within 48 hours of symptom onset.
The CDC discourages widespread use of antivirals to prevent influenza, either pre- or postexposure, although it specifies certain situations in which usage would be acceptable (TABLE 2).5 There is some concern that widespread use could lead to the emergence of drug-resistant strains and that using postexposure dosing could lead to suboptimal treatment if influenza infection occurred before the start of prophylaxis. If postexposure antivirals are prescribed, they should be started within 48 hours of exposure and continued for 7 days after the last exposure.
Continue to: A potential perfect storm