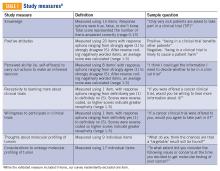
Clinical trial perceptions included questions assessing (1) patient knowledge about trials; (2) patient attitudes toward trials; (3) perceived ability (ie, self-efficacy) to carry out actions involved in making an informed decision about trial participation; (4) receptivity to learning more about trials; and (5) willingness to participate in trials. These outcome measures had been previously developed and pilot tested for reliability and validity (TABLE 1).6
Thoughts about molecular profiling of tumors were assessed using nine items (TABLE 1). Of these, items assessing potential benefit or harm of molecular profiling were assessed using a 7-step Likert scale. Items assessing maximal benefit or harm of therapy, importance of quality vs length of life, and concern about the cost of molecular testing were assessed using a 5-step Likert scale. The study team developed and piloted these questions because there is no validated survey assessing these domains.
Considerations to undergo molecular profiling were assessed using 17 items. Items were in response to the question, “To what extent did you consider the following issues or concerns at the time you decided to get molecular testing of your cancer?” Responses were assessed using a 5-point Likert scale.
Data Collection
Patients 18 years and older evaluated at NM between November 20, 2012, and November 20, 2017, with a diagnosis of sarcoma were identified by query of the NMEDW by ICD-10 codes (C40, C41.9, C44.99, C45-49, C55, C71.9, D48, D49.9, and M12.20) or equivalent ICD-9 codes. Patients were subsequently excluded if they did not have a diagnosis of bone or soft tissue sarcoma, no e-mail address listed, had died, or had not been evaluated at an NM clinic in the previous 5 years. Patients with a diagnosis of gastrointestinal stromal tumor and Kaposi’s sarcoma were also excluded.
A personalized contact e-mail was sent to patients containing an explanation of the survey and an internet link to the electronic survey through REDCap from January 2018 to March 2018. If patients did not respond to the survey, two follow-up reminder e-mails were sent 2 and 4 days following the initial survey. The link was protected so that each patient could complete the survey only once. Responses were collected through the REDCap platform. Patients read and signed an electronic consent form prior to completing the survey.
Upon completion of the survey, patients were offered a $50 VISA gift card as compensation, with an option to donate their compensation to the Robert H. Lurie Comprehensive Cancer Center Sarcoma Research Fund.
Over the described survey period, open clinical trials for patients with bone and soft tissue sarcoma available at NM were evaluated. The number of patients screened and accrued to each trial were recorded.