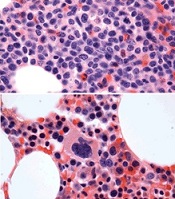
before (top) and after 7 weeks
of treatment (bottom)
© Knoechel et al.
Results of a case study suggest a gamma-secretase inhibitor (GSI) can be effective against Notch-mutated acute lymphoblastic leukemia (ALL).
The patient, who had early T-cell precursor ALL (ETP-ALL), achieved a complete hematologic response to treatment with BMS-906024, a GSI with anti-Notch
activity.
The patient was then able to proceed to hematopoietic stem cell transplant and was leukemia-free at last follow-up.
The researchers said this suggests that GSIs might hold promise for treating ALL and other cancers characterized by Notch mutations.
Birgit Knoechel, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues described this case study in Cold Spring Harbor Molecular Case Studies.
The patient was a 53-year-old male with ETP-ALL who had failed previous rounds of chemotherapy and was then enrolled in a clinical trial of BMS-906024.
The patient began to show immediate improvement after starting treatment with the GSI. After 3 cycles, he went on to transplant and has since been leukemia-free—for 19 months so far.
To determine the genetic basis for the patient’s response to BMS-906024, researchers performed targeted and whole-exome sequencing on his leukemic cells.
They identified 4 potential mutations driving disease progression, including a novel mutation in the NOTCH1 gene that resulted in hyperactive signaling. This mutated gene copy was also duplicated in the cancer genome, resulting in elevated expression.
However, the NOTCH1 mutation, along with 2 of the other mutations, were absent in the remission bone marrow.
The researchers also cultured the patient’s leukemic cells to determine the molecular response to treatment.
Cells treated with BMS-906024 had greatly reduced levels of mutated NOTCH1 protein. RNA sequencing demonstrated that Notch target genes were sensitive to the treatment.
The MYC oncogene, on the other hand, was not sensitive to BMS-906024.
Epigenetic analysis revealed that the enhancer driving MYC expression in the leukemic cells was not Notch-dependent, but rather BRD4-dependent, suggesting another possible therapeutic option for MYC-expressing tumors.


