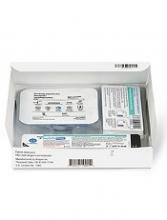
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a label variation for Neulasta® (pegfilgrastim) to include the Neulasta® Onpro® Kit.
The Neulasta Onpro Kit is an on-body injector (OBI) delivery system for Neulasta, which is approved in the European Union to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults treated with cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukemia and myelodysplastic syndromes).
The Neulasta Onpro Kit includes a specifically designed Neulasta pre-filled syringe along with a single-use OBI. The small, lightweight OBI is applied to a patient’s skin on the same day of chemotherapy.
The OBI is intended to facilitate timed delivery of the correct dose of Neulasta and eliminate the need for patients to return to a healthcare setting the day after they receive chemotherapy.
The CHMP’s opinion on the Neulasta Onpro Kit will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the centralized marketing authorization of Neulasta will be updated to include information on the Neulasta Onpro Kit in the drug’s label.
This change will be valid throughout the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions about Neulasta’s label on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.