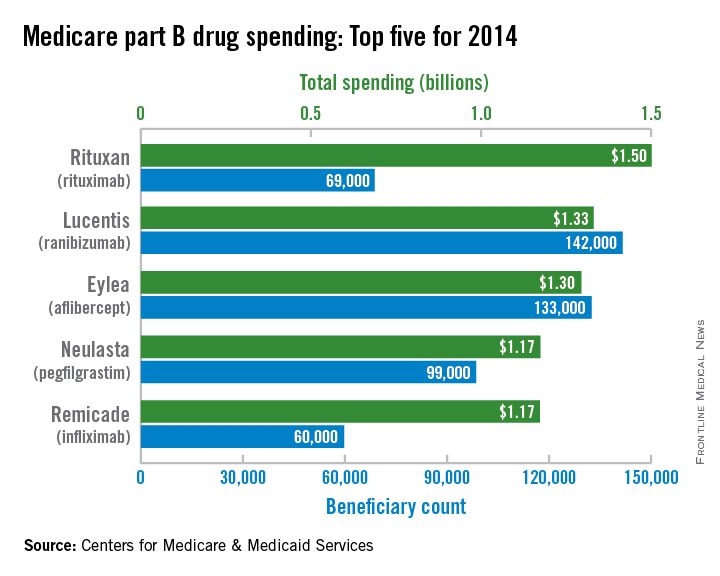
In 2014, Medicare part B drug spending was led by the $1.5 billion cost of Rituxan (rituximab), the Centers for Medicare & Medicaid Services reported.
That made Rituxan – approved to treat non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis – one of six drugs to exceed $1 billion in spending by Medicare part B for the year. Second and third on the list were the macular degeneration/macular edema/diabetic retinopathy drugs Lucentis (ranibizumab) at $1.33 billion and Eylea (aflibercept) at $1.3 billion, according to the Medicare drug spending dashboard.
The other three members of the part B $1-billion club were Neulasta (pegfilgrastim), which prevents chemotherapy-induced neutropenia, at $1.174 billion; the rheumatoid and psoriatic arthritis/Crohn’s disease/ulcerative colitis/ankylosing spondylitis/psoriasis drug Remicade (infliximab) at $1.173 billion; and the chemotherapy drug Avastin (bevacizumab) at $1.06 billion, the CMS said.
Average cost per unit varied widely among the six drugs: Neulasta was $3,200 per unit, followed by Eylea ($960), Rituxan ($690), Lucentis ($390), Remicade ($71), and Avastin ($65).
Part B drug costs in 2014 totaled $21.5 billion for 606 different drug products, with 60% of that total cost going to the 21 drugs with spending over $250 million each, the CMS noted.