Medical affairs
This is a very important and challenging specialty area that, at its core, demands value demonstration of a medicine and communication to key stakeholders such as patients, physicians, and payers. This objective has become increasingly challenging over the past decade while evolving from a qualitative specialty to a rigorous quantitative one. Scientific and commercial success depends on efficient design, execution, and dissemination of results for real-world evidence and postapproval studies. Ideally, these data will demonstrate the medicine’s benefit-risk profile and how it fits into treatment algorithms. Communication requires leadership of physician and payer advisory boards, as well as publication planning. Close collaboration with marketing teams to advise on ethical and scientifically accurate promotional activities is another key component.
Patient safety
As the name implies, patient safety focuses on evaluating signals both from clinical trials and from literature that can accurately map out risks to patients that can arise from taking these medications. This is a critical function for proper and ethical prescription and use of medicine in today’s society. In addition to signal recognition and consultation with clinical development teams, collaboration with regulatory agencies is an important component.
Epidemiology
Epidemiologists with clinical expertise have become an increasingly important need for pharmaceutical and biotech companies over the past decade – specifically, for the design of real-world studies that demonstrate benefit-risk profiles for medicines in real-world use. These data are in demand for both private and governmental payers, as well as for regulatory agencies who are interested in evolving postapproval safety data. Successful epidemiologists often have acquired MPH degrees and expertise in study design and biostatistics.
Commercial development
Those with more financial or business acumen and clinical experience sometimes staff commercial careers. Typically commercial leads also have an MBA degree and are responsible for assessing commercial markets and forecasting and executing a path to commercial viability. Ultimately this career path can lead to a CEO position.
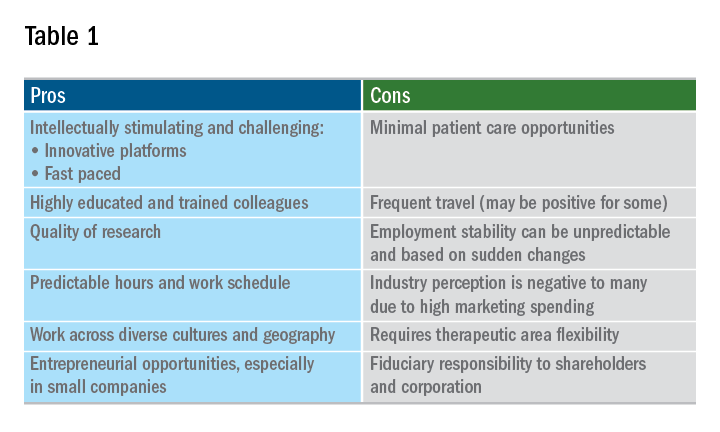
A career in industry is a perfect fit for some, but not so much for others. Table 1 outlines some pros and cons. Commercial factors do come into play with regard to corporate objectives and areas of focus. The top pharmaceutical product therapeutic categories, according to the number of drugs under development in 2017, were cancer, vaccines, diabetes, ophthalmology, gene therapy, anti-inflammatory, and antivirals and immunosuppressants; inflammatory bowel disease was 15th. Therapeutic research and development areas in gastroenterology that are relatively in demand in 2019 include IBD, irritable bowel syndrome, and nonalcoholic steatohepatitis. The high demand areas seem to change with the science and also payers’ willingness to reimburse.
Is industry a good career choice for you? Consider the following factors:
- Travel capabilities.
- Small biotech versus big pharma versus CRO.
- Capability to function in a team environment.
- Communication skills, resilience, self-awareness.
- Therapeutic area and category.
- Early stage versus late stage versus translational versus medical affairs.
- Additional education: MBA, MPH, PhD.
- Geography.
The pharmaceutical industry evolves and undergoes transformation extremely quickly in response to changes in the external environment. If you are considering a current or future career in industry, staying informed about changes in the delivery of health care and health economics is important. There is an ongoing need in industry for trainees and experienced gastroenterologists who can deploy their clinical expertise in development and communication of new medicines and devices that will make a positive difference in patients’ lives.