Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Mean duration of DM was 10 years, and mean use of basal/bolus insulin regimen was 6.1 years. Most participants (91%) used an insulin regimen containing insulin glargine and insulin aspart; the remaining participants used insulin detemir and insulin aspart. Semaglutide and liraglutide were the most commonly used GLP-1 RAs (44% and 39%, respectively) (Table 1).Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
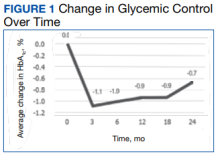
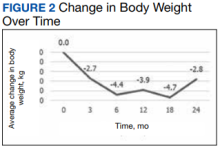
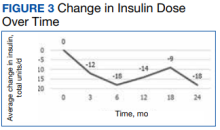
a different number of patient charts were available for review at each period (Table 2). Glycemic control was significantly improved at all time points when compared with baseline, but over time the benefit declined. The mean change in HbA1c was −1.1% (95% CI, −1.3 to −0.8; P < .001) at 3 months; −1.0% (95% CI, −1.3 to −0.7; P < .001) at 6 months; −0.9% (95% CI, −1.3 to −0.6; P < .001) at 12 months; −0.9% (95% CI, −1.4 to −0.3; P = .002) at 18 months; and −0.7% (95% CI, −1.4 to 0.1; P = .07) at 24 months (Figure 1). Mean weight decreased from baseline −2.7 kg (95% CI, −3.7 to −1.6; P < .001); −4.4 kg (95% CI −5.7 to −3.2; P < .001) at 6 months; −3.9 kg (95% CI −6.0 to −1.9; P < .001) at 12 months; −4.7 kg (95% CI −6.7 to −2.6; P < .001) at 18 months; and −2.8 kg (95% CI, −5.9 to 0.3; P = .07) at 24 months (Figure 2). Mean TDD decreased at 3 months −12 units (95% CI, −19 to −5; P < .001); −18 units (95% CI, −27 to −9; P < .001) at 6 months; −14 units (95% CI, −24 to −5; P = .004) at 12 months; −9 units (95% CI, −21 to 3; P = .15) at 18 months; and −18 units (95% CI, −43 to 5 units; P = .12) at 24 months (Figure 3). The most common AEs were hypoglycemia (30%), diarrhea (11%), nausea (4%), and abdominal pain (3%).