Results
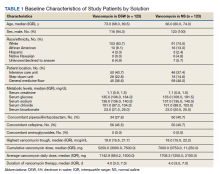
A total of 792 veterans received IV vancomycin NF/SGVHS in the defined study period. Of these, 381 veterans were excluded, including having < 80% of doses in a single solution (213 veterans), receiving IV vancomycin for < 48 hours (149 veterans), and not having necessary laboratory data available to assess a change in kidney function (19 veterans). An additional 165 veterans were randomly excluded from the D5W cohort in order to have an equal comparison group to the NS cohort; therefore, a total of 246 veterans were included in the final assessment (123 veterans in each cohort). The median patient age was 73 years (IQR, 68.0, 80.5) in the D5W group and 66 years (IQR, 60.0, 74.0) in the NS group; 83.7% of veterans in the D5W group and 74% veterans in the NS group were white; 94.3% of the D5W group and 100% of the NS group were male (Table 1).
Adverse Effects by Solution
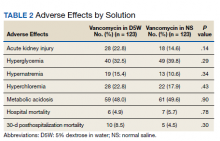
The percentage of AKI in the D5W group was 22.8% compared with 14.6% in the NS group (P = .14), and all cases were classified as stage 1 AKI. Baseline cases of hyperglycemia, hypernatremia, hyperchloremia, or metabolic acidosis were not included in the reported rates of each in order to determine a change during vancomycin therapy (Table 2).
The percentage of patients with hyperglycemia in the D5W group was 32.5% compared with 39.8% in the NS group (P = .29). The percentage of patients with hypernatremia in the D5W group was 15.4% compared with 10.6% in the NS group (P = .34). The percentage of patients with hyperchloremia in the D5W group was 22.8% compared with 17.9% in the NS group (P = .43). The percentage of patients with metabolic acidosis in the D5W group was 48.0% compared with 49.6% in the NS group (P = .90).
There were no significant differences in either in-hospital or posthospital mortality between the D5W and NS groups (in-hospital: 4.9% vs 5.7%, respectively; P = .78; 30-day posthospitalization: 8.5% vs 4.5%, respectively; P = .30).
Discussion
This retrospective cohort study comparing the AEs of vancomycin diluted in NS and vancomycin diluted with D5W showed no statistically significant differences in the incidence of AKI or any metabolic AEs. Although these results did not show an association between the incidence of AEs and the dilution fluid for vancomycin, other factors may contribute to the overall incidence of AEs. Factors such as cumulative vancomycin dose, duration of therapy, and presence of concomitant nephrotoxins have been known to increase the incidence of AKI and may have a greater impact on this incidence than the fluid used in administering the vancomycin.
These results specifically the incidence of AKI were not consistent with previous trials evaluating the AEs of NS. Based on previous trials, we expected the vancomycin in the NS cohort to have a significantly higher incidence of hypernatremia, hyperchloremia, and AKI. Our results may indicate that the volume of crystalloid received played a greater role on the incidence of AEs. Our study assessed the effect of a diluent for one IV medication that may have been only a few hundred milliliters of fluid per day. The total volume of IV fluid received from vancomycin was not assessed; thus, it is not known how the volume of fluid may have impacted the results.
One consideration with this study is the method used for monitoring vancomycin levels. Most of the patients included in this study were admitted prior to the release of the updated vancomycin guidelines, which advocated for the transition from traditional trough-only monitoring to AUC/MIC. In September 2019, NF/SGVHS ICUs made the transition to this new method of monitoring with a hospital-wide transition following the study end date. The D5W group had a slightly higher percentage of patients admitted to the ICU, thus were more likely to be monitored using AUC/MIC during this period. Literature has shown the AUC/MIC method of monitoring can result in a decreased daily dose, decreased trough levels, and decreased incidence of nephrotoxicity.11-14 Although the method for monitoring vancomycin has the potential to affect the incidence of AKI, the majority of patients were monitored using the traditional trough-only method with similar trough levels reported in both groups.
Limitations
This study is limited by its retrospective nature, the potential introduction of biases, and the inability to control for confounders that may have influenced the incidence of AEs. Potential confounders present in this study included the use of concomitant nephrotoxic medications, vancomycin dose, and underlying conditions, as these could have impacted the overall incidence of AEs.