Results
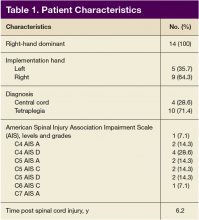
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
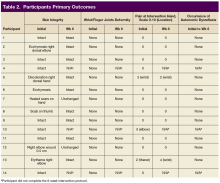
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
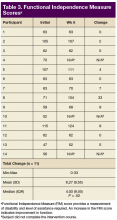
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.