In vitro studies have demonstrated that NIs are less likely to select for mutations compared with NNIs and PIs. The NNIs have limited genotypic coverage and have a lower barrier to resistance. Strategies for targeting HCV proteins include using a NI NS5B protein inhibitor as the backbone with a high barrier to resistance in combination with 1 or 2 other DAAs with lower barriers to resistance, or the combination of 3 DAAs with lower barriers to resistance. 11 Ribavirin has broad-spectrum antiviral activity, one of which is anti-HCV activity. The mode of action of RBV against HCV is not well understood, but several mechanisms have been proposed, one of which is via inhibition of viral-dependent RNA polymerase.
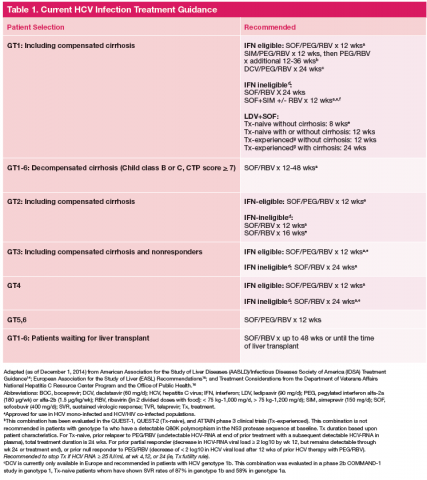
Current HCV Treatment Recommendations
Current treatment recommendations are available from the American Association for the Study of the Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) (http://www.hcvguidelines.org); the VA National Hepatitis C Resource Center Program and Office of Public Health (http://www.hepatitis.va.gov/pdf/2014hcv.pdf); and the European Association for the Study of the Liver (EASL) (http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014). These recommendations are updated frequently as new drugs enter the marketplace (Table 1). 14-16
Since most patients are unable or unwilling to tolerate interferon, the majority of patients in treatment are currently receiving interferon-free combinations. Treatment of genotype 1 is currently dominated by the offlabel use of sofosbuvir in combination with simeprevir. Data have been published from a single phase II trial in patients with and without cirrhosis. 7 Of note are emerging data from observational studies confirming the efficacy of over 80% SVR in patients with cirrhosis and genotype 1a with or without prior treatment. 17 Treatment of patients with genotype 2 infection is dominated by the use of sofosbuvir and RBV combination. However, patients with cirrhosis do not respond as well as patients without cirrhosis, and it remains to be seen whether extending therapy is of any benefit.
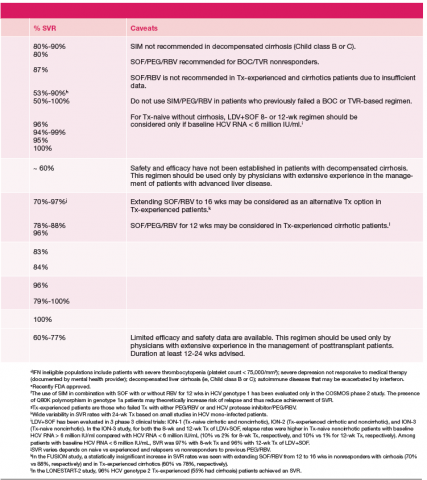
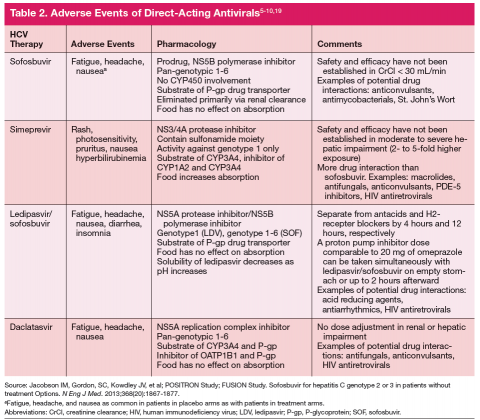
One exception for the use of PEG is the recommendation for patients with HCV genotype 3 and cirrhosis to consider the combination of PEG, RBV, and sofosbuvir for 12 weeks. 18 This has the advantage of being more effective, less costly, and shorter treatment duration compared with the sofosbuvir and RBV association but is only appropriate for patients who can tolerate interferon. Common AEs and potential contraindications to treatment are listed in Table 2. 5-10,19
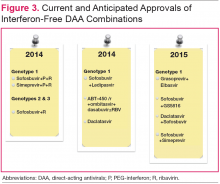
New Treatment Options for HCV in 2015
Figure 3 details DAA combinations currently in phase III trials that are expected to receive approval in 2015. This will expand the repertoire of drug combinations available and enable fine-tuning of regimens according to patient and viral characteristics and cost requirements.
Cost and Effectiveness
Cost-effectiveness issues are of immediate importance for health care systems and payors. The first generation PIs, boceprevir and telaprevir, were used in combination with PEG and RBV and entailed pharmacy costs on par with current noninterferon regimens. 20,21 Studies generally demonstrated that these treatments are cost-effective, especially in patients with advanced fibrosis. Ollendorf and colleagues recently published an analysis of the costs of using interferon-free regimens for treatment of 540,000 patients with chronic HCV in California. 22 Assuming that 50% of patients would present for treatment, the cost of the new DAAs would be immense and result in an increase in costs from $12 billion to $34 billion in the first year and net costs of $6 billion by the 20th year. If treatment were limited to patients with advanced cirrhosis, the first year costs would be increased by $7 billion, but at 20 years there would be approximately $1 billion in net cost savings.