Both dolutegravir and raltegravir can be taken with or without food. However, if the patient is taking a divalent cation (magnesium, iron, or calcium) then the medications must be separated, causing the patient to take medications multiple times per day, or taken with food (and lose the benefit of with or without food).
Cost and the New Era of ARV Generics
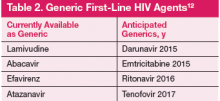
Previously, the majority of HIV regimens were similarly priced and had little impact on prescribing practices. Several ARV are now available as generics. In the next 2 years, generics may have major impact on prescribing practice as first-line options (darunavir, atazanavir, and efavirenz) will be available as generics (Table 2). 12
Prior authorizations are common for combination tablets, for example: tenofovir/emtricitabine (coformulated as Truvada). Some companies are restricting the medication to HIV treatment, and excluding HIV preexposure prophylaxis (PrEP) as an indication. By 2017, both agents recommended for PrEP will be generic. This may expand the utilization of PrEP secondary to reduced cost. Patent expirations will make medications cheaper, though likely less convenient. Unfortunately, providers may no longer have the freedom to choose coformulated tablets as entire regimens become available as generic. Currently many drug manufacturers will provide coformulated options through patient assistance programs if there is a denial for coverage by the insurance provider. Only time will tell if patients will have the option of one-pill-a-day regimens or if 3 to 5 generic tablets daily will become the new norm.Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Health and Human Services, the Indian Health Services, or the U.S. Government. Please review complete prescribing information for specific drugs or drug combinations—including but not limited to indications, contraindications, warnings, adverse effects, and drug interactions- before administering pharmacologic therapy to patients.
Journal Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combination—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.