Outcomes and Data Collection
For evaluation of the primary outcome (2-year overall hospitalization risk), the number of unique mental health and medical/surgical hospitalizations was identified by the number of discharge summaries documented in the patient chart during the evaluation period. Time to first hospitalization was recorded for the survival data analysis. Secondary outcomes were mental health hospitalization risk, medical/surgical hospitalization risk, and all-cause mortality within 2 years.
Demographic data that were collected included age, sex, comorbid mental health disorders, comorbid SUDs, and concomitant use of psychotropic medications at index date (baseline). Select comorbid mental health disorders (anxiety, schizophrenia, depression, bipolar disorder) and substance use disorders (alcohol, opioid, illicit drug) also were identified. Data on insomnia and pain comorbidities (headaches or migraines; neuropathy; head, neck, back, arthritis, or joint pain) were collected, as these comorbidities could be indications for prescribing benzodiazepines and opioids. Concomitant baseline use of classes of psychotropic medications (antipsychotics, non-SSRI/SNRI antidepressants, mood stabilizers, anxiolytics, nonbenzodiazepine sedatives/hypnotics) also were documented. Last, hospitalizations within 6 months before the initial SSRI/SNRI prescription date were noted.
Statistical Analysis
Descriptive statistics were used to analyze all baseline demographic data. Continuous measures were evaluated with 1-way analyses of variance and post hoc Bonferroni-corrected pairwise comparisons, and categorical measures with contingency tables and χ2 tests or Fisher exact tests. When the overall χ2 test was significant across all 4 study groups, post hoc comparisons were performed between the SSRI/SNRI monotherapy group and each other group with Bonferroni adjusted for 3 comparisons.
Unadjusted and adjusted Weibull proportional hazard regression models were used to estimate hospitalization risk within 2 years after the index date for the different study groups with the SSRI/SNRI monotherapy group as the referent. Robust standard errors were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The Weibull model (and not the Cox model) was used because it does not assume hazard remains constant over time, which is appropriate in this instance, as the risk of an adverse event (AE) may be higher when first starting a medication or combination of medications relative to when doses are stabilized. Models were adjusted for age, sex, baseline mental health disorders, and baseline psychotropic medications. As earlier hospitalizations showed evidence of effect modification when this covariate was tested, hazard analyses were limited to patients not previously hospitalized.
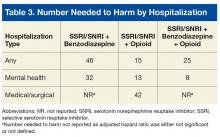
The effect size of differences in hospitalization risk meeting statistical significance was assessed by estimating the number needed to harm (NNH) and 95% CIs (not shown) to observe 1 additional hospitalization in each medication group relative to the SSRI/SNRI monotherapy group over a 90-day period. A 95% CI for NNH that did not include 0 indicated the NNH was significant at the .05 level.10 All-cause mortality was evaluated with the Fisher exact test with post hoc Bonferroni-corrected comparisons as appropriate.
Results
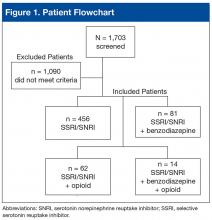
Of 1,703 patients screened, 613 met all study inclusion criteria (Figure 1).
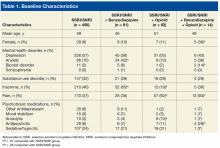
Most excluded patients had been prescribed an SSRI or SNRI by a non-VA provider or another VA facility and were transferring care to SAVAHCS; they were not true “new starts” on an SSRI or SNRI for PTSD.Baseline characteristics revealed no significant differences between groups in age or comorbid depression, schizophrenia, or SUDs (Table 1).
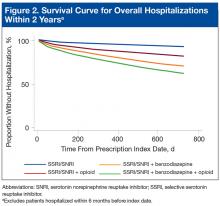
Concomitant use of a non-SSRI/SNRI antidepressant and a mood stabilizer was also similar across groups. Rates of anxiety and insomnia were higher in the SSRI/SNRI and benzodiazepine therapy group than in the SSRI/SNRI monotherapy group. As expected, rates of comorbid pain were higher in the 2 groups on concurrent opioid therapy. The proportion of female patients and the incidence of bipolar disorder and antipsychotic use were higher in the SSRI/SNRI, benzodiazepine, and opioid therapy group. One-fourth to one-third of patients across all study groups had an active diagnosis of a select SUD.With the SSRI/SNRI monotherapy group as the referent, all concurrent therapy groups were at significantly increased risk for overall hospitalization within 2 years after the index date (Tables 2 & 3, Figure 2).
The SSRI/SNRI and benzodiazepine therapy group had an adjusted HR (AHR) of 2.6 (95% CI, 1.1-5.7) and an NNH of 46; the SSRI/SNRI and opioid therapy group had an AHR of 6.1 (95% CI, 2.6-14.0) and an NNH of 15; and the SSRI/SNRI, benzodiazepine, and opioid therapy group had an AHR of 3.9 (95% CI, 1.1-14.6) and an NNH of 25.Risk for mental health hospitalization was significantly increased in all concurrent therapy groups relative to the referent group.
The SSRI/SNRI and benzodiazepine therapy group had an AHR of 5.5 (95% CI, 1.6-18.7) and an NNH of 32; the SSRI/SNRI and opioid therapy group had an AHR of 12.3 (95% CI, 3.3-46.2) and an NNH of 13; and the SSRI/SNRI, benzodiazepine, and opioid therapy group had an AHR of 20.0 (95% CI, 4.0-101) and an NNH of 8.Although the risk for medical/surgical hospitalization was not significantly increased in the SSRI/SNRI and benzodiazepine therapy group (AHR, 1.9; 95% CI, 0.67-5.6), a significant difference was found in the SSRI/SNRI and opioid therapy group (AHR, 4.4; 95% CI, 1.6-12.0; NNH, 42).
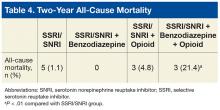
After the patients who were hospitalized within 6 months before the index date in the SSRI/SNRI, benzodiazepine, and opioid therapy group were excluded, there were no medical/surgical hospitalizations. The overall cohort’s 2-year all-cause mortality was significantly higher (P < .01) in the SSRI/SNRI, benzodiazepine and opioid therapy group (21.4%) than in the SSRI/SNRI monotherapy group (1.1%) (Table 4).