Photo Rounds
Jeff Hall, MD
Curt Elliott, MD
University of South Carolina Department of Family and Preventive Medicine, Columbia (Dr. Hall); Yangqu County Hospital, Shanxi Province, China (Dr. Elliott)
jeff.hall@uscmed.sc.edu
The authors reported no potential conflict of interest relevant to this article.

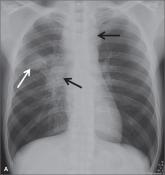
CASE › Mr. J’s clinical history and chest x-ray findings are highly suggestive of active TB. It was not unreasonable to initially treat him for a bacterial pneumonia, although fluoroquinolones should be used cautiously in this setting, because they are one of the most effective second-line drugs for TB, and using them as a single agent will often invoke drug resistance. Because he failed to respond to treatment for bacterial pneumonia and his presentation suggests TB or another serious cause of nonresponsiveness to standard treatment for community-acquired pneumonia (CAP), you admit him to the hospital.
Treatment for active TB requires multiple drugs in 2 phases
While all family physicians should suspect active TB in appropriate clinical situations and be comfortable with obtaining cultures and initiating empiric treatment, most will want to seek consultation with an infectious disease (ID) specialist especially in the scenarios listed in TABLE 2.5,22 Delayed or inappropriate treatment of active TB remains a major public health problem and cause of multidrug-resistant TB. Inappropriate treatment has been shown to be associated with a 27-fold increase in treatment failure.23 TB treatment guidelines are available from the CDC,24 World Health Organization,25 and International Union Against Tuberculosis and Lung Disease.26
In the initial phase of treatment for active TB, patients should begin 4 drugs—INH, RIF, EMB, and PZA—for 2 months. Appropriate treatment requires the use of multiple medications administered in 2 phases. In the initial phase, a patient with suspected TB should begin 4 drugs—usually INH, RIF, ethambutol (EMB), and pyrazinamide (PZA)—for 2 months.1,2,27 The daily pediatric and adult doses and common adverse effects of these medications are summarized in TABLE 3.28 Although most cases of TB can be adequately treated with 2 drugs to which the organism is susceptible, 4 drugs are used initially while awaiting drug sensitivity test results because of the risk of inadequately treating a strain of drug-resistant TB. Before beginning these medications, a chest x-ray, LFTs, HIV antibody test, hepatitis B and C serologies, a serum creatinine, and complete blood count should be obtained in all patients.5 If EMB is prescribed, the patient should also undergo testing for red-green color discrimination, because red-green color vision disturbance is a potential adverse effect of this medication.
All 4 drugs may be administered as a single daily dose, and may be taken together.29 They are ordinarily given either daily for 8 weeks, or daily for 2 weeks followed by a twice-weekly schedule for the remaining 6 weeks in higher doses, although the twice-weekly dose of RIF is the same as the daily dose. All are pregnancy category C, although for active TB, the benefit of treatment is almost always greater than the potential harm.
The continuation phase of treatment starts at 8 weeks, when the results of initial cultures and drug sensitivity tests should be available to guide therapy. A second set of cultures and AFB smears is obtained at 8 weeks to document clearing of the initial infection and guide duration of the continuation phase. If the initial culture was positive for Mycobacterium tuberculosis and the organism was sensitive to both INH and RIF, these 2 drugs should be continued for another 4 months (for a total of 6 months of treatment). PZA and EMB may be stopped at 2 months if the organism is sensitive to both INH and RIF. Thus, for most patients with active TB, the standard regimen will be 4 drugs for 2 months, then 2 drugs for 4 months.2
When should the standard treatment regimen be modified?
If a patient with active TB has persistently positive cultures and cavitary disease on an initial chest x-ray, treatment should be extended to 9 months. If the second set of cultures obtained 2 months after beginning drug treatment is positive and there was cavitary disease on the initial chest x-ray, the continuation phase should be extended by 7 months (for a total of 9 months of treatment).30 If a patient has either cavitary disease or persistently positive cultures (but not both), then the length of therapy is determined on an individual basis in consultation with an ID specialist.
Should a patient’s cultures show resistance to any of the first-line drugs, obtain consultation with an ID specialist. Treatment of multidrug-resistant TB (resistant to INH and RIF) and its subset, extensively drug-resistant TB (resistant to INH and RIF, plus any fluoroquinolone, plus either an aminoglycoside or capreomycin) requires prolonged courses of therapy with multiple drugs administered by DOT.31,32
If at any point during treatment a patient shows clinical deterioration that’s believed to be due to a resurgence of his or her TB disease, obtain a new set of cultures and, in consultation with an ID specialist, add at least 2 drugs to which the patient has not been exposed. Never add only one drug to a failing regimen; active TB always requires 2 drugs to cure, and the patient may have developed resistance to all of the drugs he or she is currently receiving.