• Use a validated breast cancer risk assessment tool for any woman with a suspicious family history, precancerous breast lesions, or reproductive risk factors. C
• Recommend a semi-annual clinical breast exam and an annual mammogram for women at high risk for invasive breast cancer. C
• Discuss chemoprevention with a selective estrogen-receptor modifier or aromatase inhibitor with women at high risk for breast cancer and low risk for adverse events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Identifying patients at risk
Among the known risk factors for breast cancer, some are modifiable (use of oral contraceptives and alcohol consumption, for example); others, such as family history and age at which menopause occurs, are not (TABLE 1).4-7 Aging itself confers the greatest risk: The incidence of breast cancer comes close to doubling at each 10-year interval before menopause and continues to climb, but more slowly, thereafter.8,9
TABLE 1
Risk factors for breast cancer4-7
| Nonmodifiable | Age, atypical hyperplasia, chest wall radiation (between the ages of 10-30 y), early menarche, family history, late menopause, race, sex |
| Modifiable | Alcohol consumption, hormone therapy (for menopausal symptoms, oral contraceptives), obesity, parity (first child after age 35, nulliparity) |
Estrogen exposure: The risk is cumulative
A number of studies have linked early onset of menarche (<12 years of age) and late menopause (>55 years) to an increase in breast cancer risk. Nulliparity, or having a first child after age 35, is also associated with greater risk; oophorectomy prior to age 50 may reduce the risk by as much as 40%.4,5,10-13
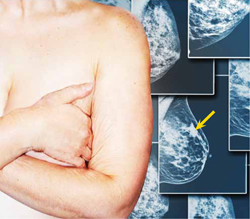
The mammogram shows a malignancy in the superior portion of the breast (arrow). Oral contraceptive use is an additional risk, but the effect slowly diminishes in the 10 years after cessation.4,5 Postmenopausal hormone replacement therapy—specifically, oral conjugated equine estrogen and medroxyprogesterone acetate—was found by the Woman’s Health Initiative to increase breast cancer risk.6
Other nongenetic risk factors include:
Atypical findings on breast biopsy. Evidence of atypical ductal hyperplasia (ADH) or lobular hyperplasia (ALH) is associated with a 4-fold increase in risk.7
Environmental exposure. Radiation, especially to the chest wall (typically as a treatment for Hodgkin’s lymphoma) increases a woman’s risk for breast cancer, particularly if the exposure occurred when she was between the ages of 10 and 30.14
Lifestyle factors. Obesity, particularly in postmenopausal women, and alcohol consumption of more than a drink or two per day are both associated with an increased risk.4
Genetic mutations and breast cancer risk
An estimated 5% to 10% of breast cancers are inherited.5 Genetic susceptibility is generally transmitted as an autosomal dominant trait.
There are 2 known breast cancer genes, BRCA1 and BRCA2, located on the long arm of chromosomes 17 and 13, respectively. The genes themselves encode tumor suppressor proteins. Mutations in these genes impair the DNA repair process, resulting in increased risk.8
The chance of carrying a mutation in either BRCA1 or BRCA2 is estimated at one in 500 to 800 in women of Northern/Western European descent. Among Ashkenazi Jews, however, the frequency is about one in 50.5
A thorough family history that takes into account both the number of affected relatives and their age at diagnosis (TABLE 2)8,15 is helpful in determining whether a patient is at low, high, or very high risk of carrying a genetic mutation. Women who have no first-degree relative with breast cancer—or a relative who was diagnosed with breast cancer after age 50—are at low risk, while those with at least one first-degree relative diagnosed with breast cancer before the age of 50 would be categorized as high risk.
A woman with a family history of early-onset breast or ovarian cancer or a relative who developed both breast and ovarian cancer, bilateral breast cancer, or male breast cancer would be classified as very high risk for a genetic mutation, as would a patient with 2 or more family members affected by breast or ovarian cancer.
Ashkenazi Jewish heritage and a relative who was diagnosed with ovarian or breast cancer indicate an increased likelihood of a BRCA mutation, as well.8 (Other genetic conditions, with mutations that are distinct from the BRCA genes, have also been linked to breast cancer, but occur less frequently.)
BRCA gene testing can confirm very high risk status, prompting the initiation of preventive measures and facilitating early detection. Such testing can also identify—and relieve the anxiety of—noncarriers in high-risk families. Recently published guidelines from the US Preventive Services Task Force (USPSTF) support testing in women with suspicious family histories with a grade B recommendation, indicating that there is at least fair evidence that testing improves important health outcomes and that the benefits of testing outweigh the harms.15

