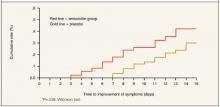
Figure
Kaplan-Meier curve for improvement—amoxicillin (n=67) vs placebo (n=68)*
TABLE 2
Comparison of mean Likert scores by group across follow-up time points Question asked at each time point:
| “On a scale of 1 to 10, How sick do you feel today?”* | |||
|---|---|---|---|
| Time† | Amoxicillin (n=67) | Placebo (n=68) | P value |
| Day 0 (SD) | 6.10 (2.0) | 6.30 (1.9) | NS |
| Day 3 (SD) | 4.33 (1.8) | 4.73 (1.9) | NS |
| Day 7 (SD) | 3.15 (2.1) | 3.30 (2.0) | NS |
| Day 14 (SD) | 2.30 (1.9) | 2.80 (2.5) | NS |
| Likert score of 1 represents “perfect health” to 10 representing “worst condition.” | |||
| * Statistical tests—Orthogonal contrasts. | |||
| † Data shown represent mean and standard deviation (SD). | |||
TABLE 3
Mean number of days to improvement by group and number of signs and symptoms (at baseline) for patients who improved
| Number of signs and symptoms | Amoxicillin (n=32) | Placebo (n=25) |
|---|---|---|
| (1) Mean (n, SD) | 7.8 days (16, 3.7) | 11.0 days (10, 2.6) |
| (2) Mean (n, SD) | 7.8 days (5, 3.7) | 10.3 days (6, 3.2) |
| (3–4) Mean (n, SD) | 8.6 days (11, 3.6) | 10.6 days (9, 3.0) |
| Signs and symptoms are: purulent (yellow, thick) nasal discharge predominating on 1 side, local facial pain predominating on 1 side, purulent nasal discharge on both sides, and pus in the nasal cavity. | ||
Results
During the 18-month enrollment period, the 3 providers recorded all patients aged >18 years who had at least 1 cardinal feature described by the clinical prediction rule and had symptoms for a minimum of 7 days. Thus, initially 308 patients were approached for enrollment; 173 patients did not qualify after the exclusion criteria were applied, leaving 135 patients for randomization. Sixty-seven received amoxicillin and 68 received placebo. For 11 patients in the amoxicillin arm and 8 in the placebo arm, only baseline data were collected. These patients were then considered as lost to follow-up and were counted as “not improved” in the intention-to-treat analysis.
There were no significant differences (P >.05) in baseline characteristics of the treatment groups (Table 1). Additionally, there were no significant differences in the baseline characteristics between the providers (data not shown).
In the amoxicillin group 32 (48%) had completely improved compared with 25 (37%) in the placebo group (P=.26) after 2 weeks (relative risk of treatment failure=1.3; 95% CI, 0.87–1.94). However, individuals in the amoxicillin group did improve significantly earlier, as the Kaplan-Meier curve demonstrates (Figure). The first person in the amoxicillin group improved on day 3, compared with day 7 in the placebo group. This earlier improvement continued throughout the study (P=.039).
Subgroup analysis of the 57 who demonstrated complete recovery shows the amoxicillin group improved earlier as does the Kaplan-Meier curves in the Figure. In the amoxicillin group, the median day to any improvement was day 8 compared with day 12 for the placebo group (P=.005), while the mean day to improvement for the amoxicillin group was 8.1 days vs 10.7 days for placebo group.
When patients were asked “How sick do you feel today,” the average Likert scores decreased from 6. 1 (day 0) to 2.3 (day 14), and 6.3 (day 0) to 2.8 (day 14), in the amoxicillin and placebo groups, respectively. At each time point, there were no significant clinical or statistical differences between the 2 groups in how they rated their improvement (Table 2). Furthermore, examining only those who reported total improvement within 14 days showed no differences among groups.
No statistically significant differences were observed between the treatment groups that entailed the clinical prediction rule. However, in the patients who were improved at 14 days, the average number of days to improvement was consistently between 2 to 2.5 days shorter in the amoxicillin group compared with placebo (Table 3).
Side effects
No patients dropped out of the study due to adverse side effects (Table 4). There were no serious or unexpected side effects, with the majority related to gastrointestinal problems, such as diarrhea and abdominal pain.
TABLE 4
A Frequency of reported side effects by group
| Amoxicillin Adverse effects | Placebo (n=57) | (n=59) |
|---|---|---|
| Total number of patients with any side effects | 13 | 7 |
| Diarrhea | 4 | 1 |
| Nausea | 4 | 5 |
| Emesis | 1 | 0 |
| Abdominal pain | 2 | 1 |
| Rash | 2 | 0 |
| Hot flashes | 0 | 1 |
| Jittery | 0 | 1 |
| Dizziness | 3 | 0 |
| Dry mouth | 1 | 0 |
| Vaginal infection | 2 | 0 |
| Multiple events per patient are possible. | ||
Discussion
With respect to the patient-oriented outcome of clinical improvement, amoxicillin provided no significant benefit over placebo in the treatment of patients presenting with sinusitis complaints. On average our patients who had symptoms for 11 days prior to enrollment and are typical of patients that are often recommended for treatment with antibiotics.14,15
Our findings are consistent with others in which the overall benefit of antibiotics was minimal or nonexistent.16,18 But among individuals who did improve, those who received amoxicillin improved much earlier, both clinically and statistically. Unfortunately we were not able to specify those who are likely to improve. Clearly, further patient-oriented outcome studies are needed to help primary care physicians decide which patients may benefit from antibiotic treatment.