1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines . 7th ed. Elsevier; 2017:1-15.
2. Lancet Commission on COVID-19 Vaccines; Therapeutics Task Force Members. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health . 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Me d. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med . 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol . 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun . 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems . 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med . 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines . 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med . 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med . 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; 2019nCoV-302 Study Group . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med . 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep . 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep . 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly R ep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; IVY Network ; HAIVEN Investigators . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep . 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep . 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA . Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med . 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med . 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA . 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med . 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep . 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep . 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep . 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med . 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep . 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep . 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv . 2021:12.02.21267156. doi:10.1101/2021.12.02.21267156
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q . 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA . 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; Overcoming COVID-19 Investigators ; Overcoming COVID-19 Network . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly R ep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep . 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep . 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med . 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med . 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med . 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med . 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep . 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis . 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; DMID 21-0012 Study Group . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med . 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
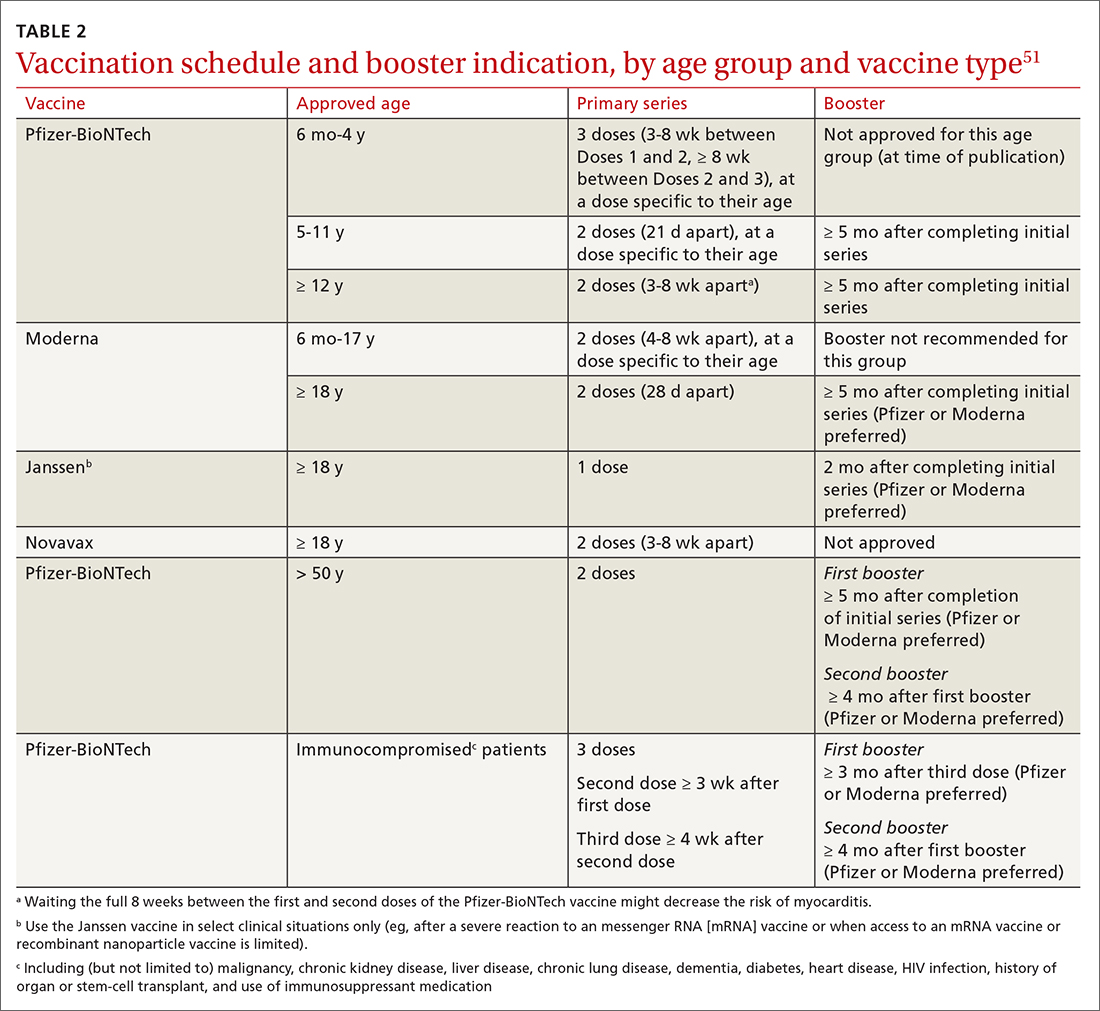
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis . 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses . 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol . 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol . 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep . 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; COVID-NET Surveillance Team . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep . 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; Overcoming Covid-19 Investigators . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med . 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics . 2021;148:e2021052336 . doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; COVID-19 Shedding Study Group . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis . 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv . 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis . 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis . 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep . 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2