Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
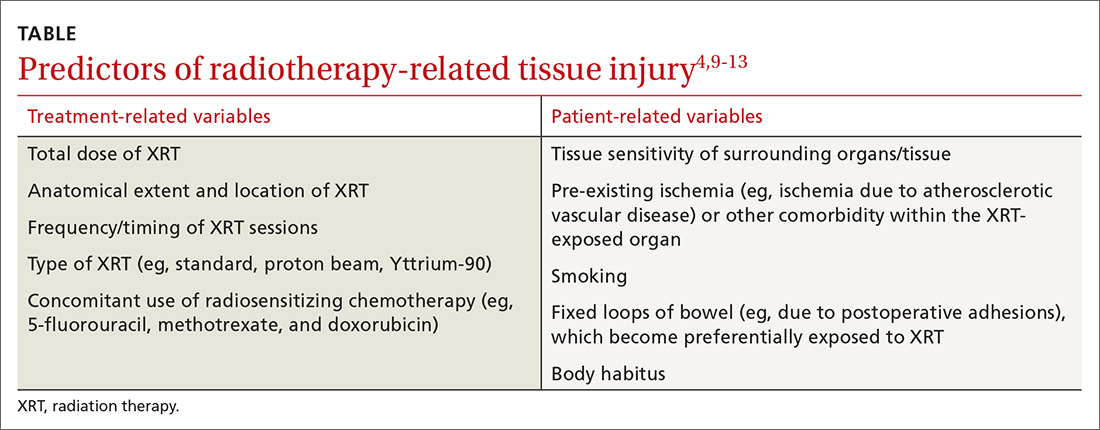
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.
Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.