It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).
Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
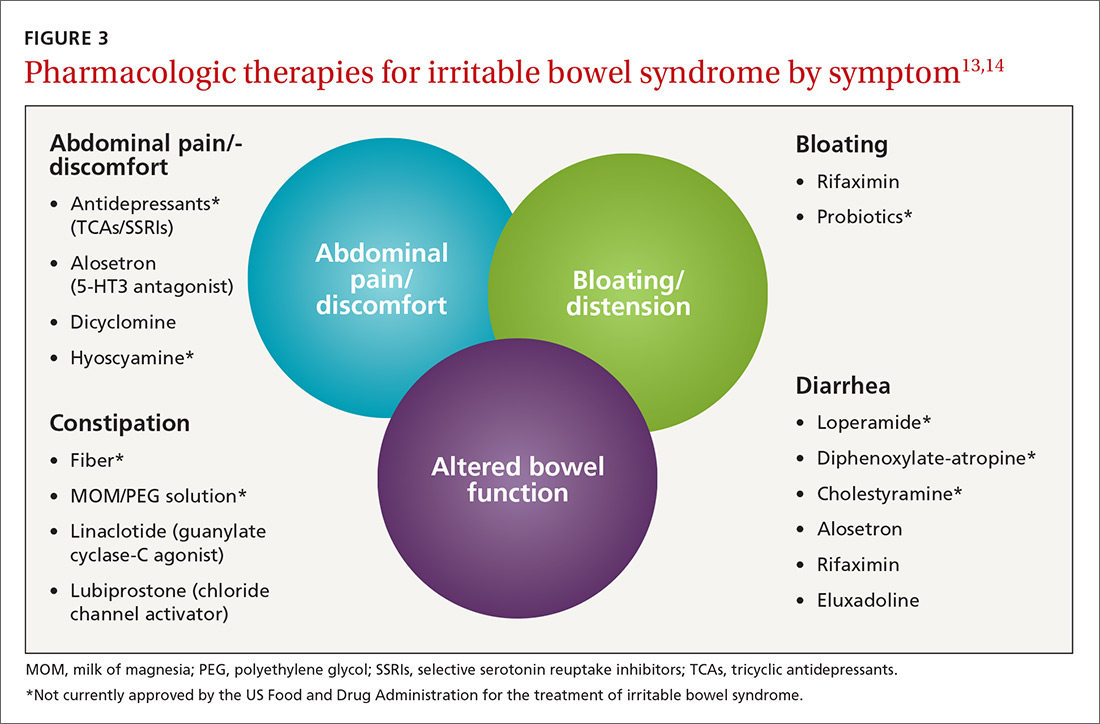
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3,13,14 including several that are commonly used and have moderate efficacy, but are not currently approved by the US Food and Drug Administration for this purpose.
Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.